Effect of Sensory Impairment on Balance Performance and Lower Limb Muscle Strength in Older Adults With Type 2 Diabetes
Article information
Abstract
Objective
To compare balance performance and lower limb muscle strength between older adults with type 2 diabetes mellitus (DM), with and without sensory impairments and non-DM groups. Influence of a number of sensory impairments, and muscle strength on balance performance were explored.
Methods
Ninety-two older adults with and without type 2 DM, were examined relative to visual function with the Snellen chart, Melbourne Edge test, and Howard-Dolman test, vestibular function with the modified Romberg test, proprioception of the big toe, and diabetic peripheral neuropathy with the Michigan Neuropathy Screening Instrument. Balance performances were evaluated with the Romberg test, Functional Reach Test (FRT), and Timed Up and Go test (TUG). Strength of knee and ankle muscles was measured.
Results
FRT of type 2 DM groups with at least two sensory impairments, was lower than the non-DM group (p<0.05). TUG of all DM groups, was worse than the non-DM group (p<0.01). Lower limb muscle strength of type 2 DM groups with two and three sensory impairments, was weaker than non-DM group (p<0.05). Regression analysis showed that type 2 DM with three sensory impairments, ankle dorsiflexors strength, and age were influential predictors of TUG.
Conclusion
There were significant differences, of muscle strength and balance performance among groups. Poorer balance and reduced lower limb strength were marked in older adults with type 2 DM, even ones without sensory impairment. Muscle weakness seemed to progress, from the distal part of lower limbs. A greater number of sensory impairments, weaker dorsiflexors, and advanced age influenced balance performance.
INTRODUCTION
Diabetes mellitus (DM), especially type 2 DM, is a major public concern of the global health community with prevalence as high as 22%–33% [1]. This condition is known to predispose physical deterioration [2]. Its vascular complications directly affect somatosensory and motor functions [3].Consequent physical impairments such as muscle weakness and balance disorder, are usually observed [4].
Multiple sensory impairments of vestibular dysfunction, visual function impairment, as well as somatosensory impairment including diabetic peripheral neuropathy (DPN) and proprioception impairment, are also common in individuals with type 2 DM [5,6]. Inputs from these sensory systems are crucial, in determining body position during balance and postural control [7]. Vestibular dysfunction leads to distorted inputs, of angular and linear motions of the body. Visual impairment, alters information of the surrounding environment. DPN causes loss of tactile and vibration sensation, and reduces proprioceptive input that leads to difficulty of ambulation. These sensory function impairments evidently result in poorer balance control, and increased risk of falls in older adults with DM, as compared to older adults without DM [4,8-10].
The number of sensory impairments was reported to have direct effects on balance performance in adults age 40 to 85 [8]. Persons with single as well as multiple sensory impairments, had different levels of difficulties related to balance and falls [8]. Herrera-Rangel et al. [5] reported multiple patterns of sensory impairments including hearing, vision, vestibular, and peripheral neuropathy, in people with type 2 DM. Fifty-two percent of their participants had at least two sensory impairments, and they were not aware of their sensory dysfunction or balance decline. However, the authors did not provide a thorough picture of sensory impairment patterns.Another study suggested that redundancy of sensory inputs available to the central nervous system was critical for balance control in challenging conditions, and multiple sensory system impairments may occur in older adults with type 2 DM even before apparent DPN [6].
Motor system function, is mainly responsive to sensory function [7]. Motor dysfunction in people with type 2 DM, was found to be responsive to vascular and nerve involvement [9]. In type 2 DM, motor impairments were associated with DPN, systemic inflammation, poor glycemic control, and duration of DM exposure as well as sarcopenia [9]. Severity of DPN was suggested to be associated with muscle strength. However, the relationship was not found with other DM complications, such as diabetic retinopathy [10]. Loss of muscle include lower density and mass, accompanied with reduced especially around ankles and knees, were common in people with type 2 DM [9]. These weaknesses consequently involve difficulty in maintaining balance and mobility, causing frequent falls in this population [4,9,11]. The association between motor dysfunction and other sensory impairments, such as visual impairment and vestibular impairment, were not documented in previous studies.
To date, no research has described the association of a number of sensory impairments, motor dysfunction, and balance performance in older adults with type 2 DM. The association of a number of sensory impairments, and lower limb muscle strength to balance performance, is also unknown. With this information, rehabilitation programs of motor and sensory functions could be recommended, to manage balance issues in this population. So, the purpose of this study was to compare balance performance and lower limb muscle strength, between older adults with type 2 DM with and without sensory impairments and non-DM control groups. The ability to predict balance performance by the number of sensory impairments and muscle strength, were also explored.
MATERIALS AND METHODS
This study was a cross-sectional, case-control comparative design.
Participants
Four hundred and fifty-nine (n=459) community dwellers older than age 60 enrolled in the study. They were included in the study if vital signs were in normal range, could understand and follow verbal instruction, and could walk independently at least for 10 minutes. They were divided into non-DM (n=212), and type 2 DM (n=247) groups (Fig. 1). Older adults were excluded if they had a history of central nervous system dysfunction such as stroke and Parkinson disease, lower limb amputation or joint replacement, lumbar or lower limb fracture and/or surgery within the previous 6 months, pain or symptoms of musculoskeletal disorders affecting movement during walking and balance performance (numeric rating pain scale increase greater than 3 out of 10) [12], and could not identify vestibular function identify by the modified Romberg test [13].

Flow diagram of enrollment the participants in this study, and divided into non-diabetes mellitus (non-DM) and type 2 DM groups. RT, Romberg test; FRT, Functional Reach Test; TUG, Timed Up and Go Test.
For the non-DM group, older adults without history of type 2 DM were excluded, if they had impaired sensory function. Visual function was identified by visual acuity score with the Snellen chart, depth perception with the Melbourne Edge test, and contrast sensitivity with the Howard-Dolman test, vestibular function with the modified Romberg test, and peripheral neuropathy. Finally, there were 20 older adults with non-DM (n=20) serving as the control group (Fig. 1).
For the groups with type 2 DM, older adults were excluded if they were diagnosed with type 2 DM less than 5 years ago. So, there were 116 older adults with type 2 DM. However, 44 of the 116 older adults with type 2 DM, declined to participate in the study. Eventually, 72 older adults with type 2 DM participated in the study (Fig. 1). Participants with type 2 DM were divided into four groups: without sensory impairments (Group 1, no visual and vestibular impairments, and DPN); with one sensory impairment (Group 2, with either visual impairment, vestibular impairment, or DPN); with two sensory impairments (Group 3, with either visual and vestibular impairment, or visual impairment and DPN, or vestibular impairment and DPN); and with three sensory impairments (Group 4, with visual and vestibular impairment and DPN), as shown in Fig. 1.
In this study, visual impairment was identified by visual acuity, contrast sensitivity, and depth perception. Binocular visual acuity was assessed, using the Snellen chart. Participants were asked to stand 3 m away from the chart, wearing their best correction lenses. The score was determined by the lowest line, and the number of correct letters participants could read, using visual acuity conversation [14]. Visual acuity score of <75 was considered visual impairment [14]. Contrast sensitivity was assessed, using the Melbourne Edge test (Australian College of Optometry, Carlton, Australia). The chart consists of 20 circles, with 25 mm diameter. The test presented a series of edges of reducing contrast with four variable orientations, i.e., horizontal, vertical, 45° left, and 45° right. Participants were asked to sit 40 cm away from the chart, wearing their correction lenses. They looked at each circle and reported the orientation verbally, or used the response key card within 20 seconds. They were asked to guess, in the case of an uncertain answer. The last edge contrast circle correctly identified, was recorded in decibel (dB) units. Values lower than 18 dB for age 60–69, 16 dB for age 70–79, and 14 dB for age 80 years and older, were considered visual impairment [15]. Depth perception was assessed, using the Howard-Dolman test (Bernell, a Division of Vision Training Products Inc., Mishawaka, IN, USA) [16]. Participants were asked to align two vertical rods in a horizontal plane from 3 m, using a robe attached on them in 20 seconds. Distance error was recorded in cm. Distance error of >2 cm was considered visual impairment. Vestibular impairment was indicated, if participants were unable to perform the modified Romberg test [13]. A trial of the modified Romberg test was used to evaluate vestibular function [13]. Participants were asked to stand with their feet together and hands at their sides for 30 seconds in four conditions including eyes open on firm surface, eyes closed on firm surface, eyes open on foam surface, and eyes closed on foam surface, respectively. The foam was medium density and 24 inches in width and length, with 4-inch height (SunMate; Dynamic System Inc., Leicester, NC, USA). Sway was defined as (1) inability to stand with feet together, (2) moving upper extremity, (3) opening eyes, (4) flexing one or both knees, toes, or heels raised from the floor, and (5) attempting to hold onto the tester, during test execution. The last condition of eyes closed on foam surface, which assesses balance relying primarily on vestibular input, was used to identify participants with vestibular dysfunction. DPN was the Michigan Neuropathy Screening Instrument (MNSI) [17] score greater than 2 out of 8, of the physical examination part, not including the monofilament test.
All participants were informed about the study, and signed an informed consent prior to participation. The study was approved by the Mahidol University Central Institutional Review Board (No. MU-CIRB 2015/035.0303).
Procedure
Participants were interviewed and tested, regarding their demographic and health information about age, sex, history of type 2 DM, and duration of DM exposure. Blood was drawn from venous by medical personnel on the day of testing to determine fasting blood sugar (FBS) and hemoglobin A1c (HbA1c). Heart rate and blood pressure were monitored. Proprioception of each participant’s big toe was tested.
Outcome measures
Participants were tested for balance performance and lower limb muscle strength as follow.
Romberg test (RT)
Participants stood with hands at side and feet together on a firm surface with eyes closed, and sway was observed for 30 seconds, for three trials [18]. Sway was defined as (1) inability to stand with feet together, (2) moving upper extremity, (3) opening eyes, (4) flexing one or both knees, toes, or heels raised from the floor, and (5) attempting to hold onto the tester, during test execution. Duration of performing without sway was the averaged value, presented in seconds.
Functional Reach Test (FRT)
Participants barefooted stood with their right side closed to a wall. They stood with feet apart at shoulder width, and hands beside the body. The right shoulder flexed at 90° with elbow extended. Location of the third metacarpal bone was recorded as a starting point. They then reached forward as far as possible without taking a step. The ending point was recorded, using the same reference point. Distance between starting and ending points was recorded as distance of reaching. Participants were allowed to practice once and performed FRT twice. Reaching distance was the averaged value presented in inches [18].
Timed Up and Go Test (TUG)
Participants sat on a 46-cm height armchair, with their back contacting the chair’s back support. They were asked to stand, walk 3 m as quickly and safely as possible, turn around, walk back, and sit down. They were allowed to use a gait assistive device, if preferred [19]. Timing was started at the command ‘go’ and stopped, when participants sat with their back touching the chair’s back support. They performed the TUG twice. The first was a practice session, and the latter, was the test [19]. Time was recorded in seconds.
The strength of knee extensors, knee flexors, ankle plantarflexors, and ankle dorsiflexors, was measured by hand-held dynamometer (Lafayette Instrument Company, Lafayette, IN, USA) [20]. All muscle groups were tested, in mid-range of joint motion. One practice trial was allowed prior to measurement of each movement. The average of three trials was recorded for each muscle group [20]. Strength was measured in kilogram.
Statistical analysis
Statistical analysis was performed using the statistical software package SPSS for Windows version 18 (SPSS Inc., Chicago, IL, USA). The Kolmogorov-Smirnov Goodness of Fit test was used to test distribution of data. Demographic data and health information were compared among groups. Chi-square test was used, to examine difference of categorical variables among groups.For continuous variables, homogeneity of variance was performed using Levene’s test. For controlling effects of age to balance performance and muscle strength, Analysis of Covariance (ANCOVA) was used, with LSD post-hoc analysis, to determine the difference among groups. A logarithmic transformation was applied to the outcome in case variance was not equal across groups before ANCOVA. The value of p<0.05 was statistically significant.
Bivariate correlations were conducted to determine the relationship between balance performance, and other independent variables in type 2 DM participants. Variables were eligible for entry into a multiple linear regression model, if they significantly correlated with balance performance. Difference in the number of sensory impairments was reported to have different levels of effect on balance performance [8]. So, variables of the number of different impairments were included in the analysis. Multicollinearity between selected independent variables was tested with the variance inflation factor (VIF). Backward linear regression analyses were used for variable selection.
RESULTS
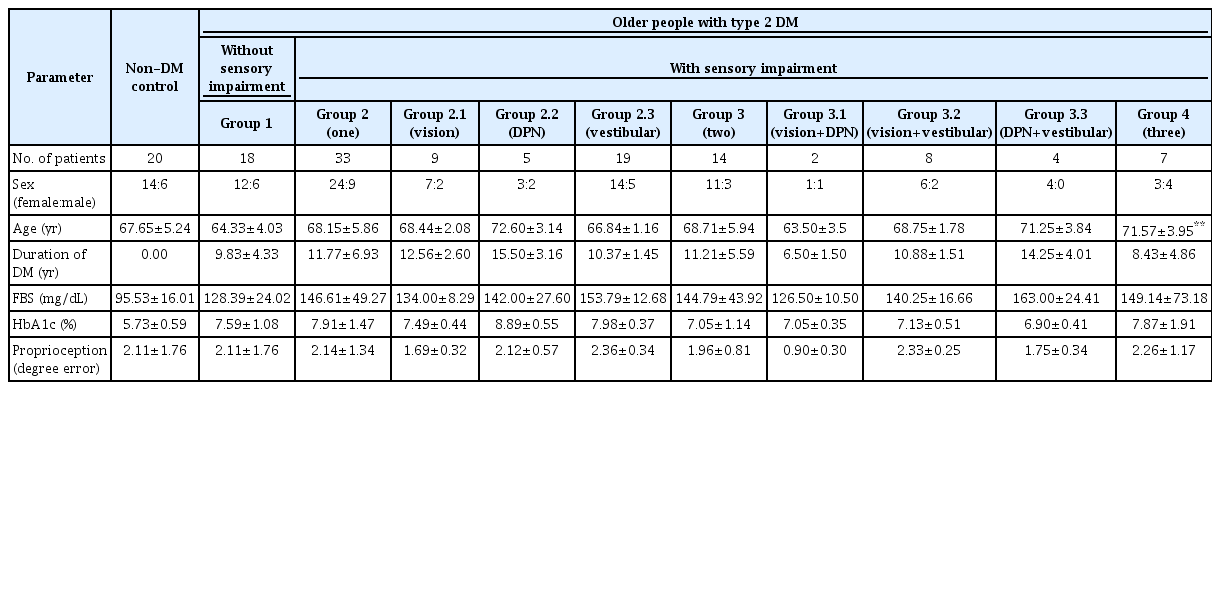
Characteristics of participants with and without type 2 DM, are presented in Table 1. The majority of participants were female, and no differences in sex proportions among groups were observed. Age was significantly higher (p=0.001) in Group 4, compared with Group 1, so all group comparisons used age as the covariate. All groups with type 2 DM had similar duration of DM exposure, level of FBS, and HbA1c. All groups had similar degree of proprioception error.
Balance performance and lower limb muscle strength of participants are shown in Table 2. After adjusted by age, TUG of all groups with type 2 DM, was significantly greater than the non-DM group (p<0.01). Among groups with type 2 DM, Group 4 had significant longer time of TUG compared to Group 1 (p=0.003), Group 2 (p=0.001) and Group 3 (p=0.035). FRT were lower for Group 3 (p=0.025) and Group 4 (p=0.014), compared to the non-DM control group. Results of RT were not different, among groups.
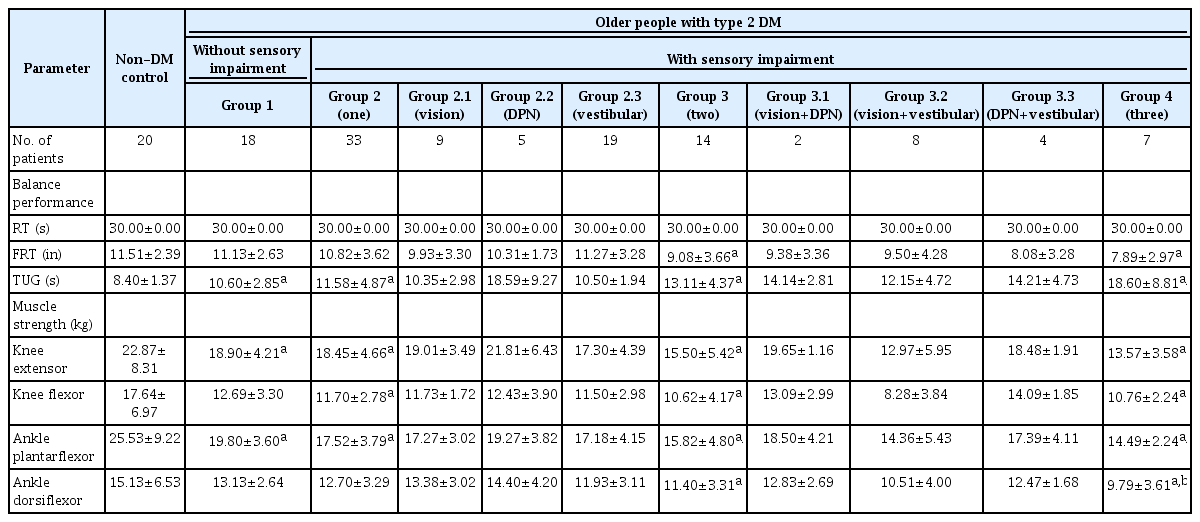
The balance performance and lower limb muscle strength of older adults with and without type 2 DM (n=92)
After adjusted by age, muscle strength of knee extensors and ankle plantarflexors of Group 1, 2, 3, and 4 were significantly different, compared with the non-DM control group (p<0.05). Knee flexors of Group 2, 3, and 4 were significantly different, compared to the non-DM control group (p<0.01). Ankle dorsiflexors were also weaker for Group 3 (p=0.020) and Group 4 (p=0.004), compared to the non-DM control group (p=0.05). Among groups with type 2 DM, ankle plantarflexors were weaker for Group 3 (p=0.045) and Group 4 (p=0.032), compared to Group 1. Ankle dorsiflexors of Group 4 was significantly weaker, compared to Group 1 (p=0.037) and Group 2 (p=0.038).
Correlation of balance performance, muscle strength, proprioceptive error, and age in participants with type 2 DM, are presented in Table 3. All lower limb muscle strength negatively correlated with TUG (p<0.05). Knee extensors correlated with FRT (p<0.05). Age also significantly correlated with FRT and TUG (p<0.05).
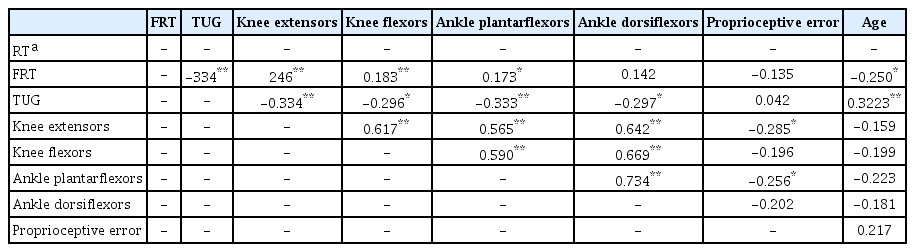
Correlation coefficients for balance performance, lower limb muscle strength, proprioception, and age of participants with type 2 DM (n=72)
Table 4 presents results of multiple linear regression analysis, for FRT and TUG. For FRT, two independent variables, i.e., DM with Group 3 and Group 4 were included in the final model. There was no collinearity, between independents variables. The model could explain 11% variance, of FRT. For TUG, three independent variables including Group 4, ankle dorsiflexors and age, were included in the final model. There was no collinearity between independent variables. The model could explain 24% variance of TUG.
DISCUSSION
This purpose of this study was to compare balance performance and lower limb muscle strength, in older adults with and without type 2 DM, with different numbers of sensory impairments. Ability to predict balance performance by numbers of sensory impairments and muscle strength, were also explored. Results showed balance impairment and weakness of lower limb muscle strength in participants with type 2 DM with difference in sensory impairments, compared with the non-DM group.
Poorer performance of FRT has been noted in people with type 2 DM with sensory impairments [21,22]. Reduced lower limb strength was associated with poorer performance of FRT in this study. Ankle plantarflexors strength was found to significantly contribute to center of mass (COM) displacement during forward reach in people with type 2 DM with 70% variance accounted [21]. Proprioception of hallux was also a significant predictor of FRT and COM displacement, in people with type 2 DM with 43% and 65% variance accounted, respectively [21]. However, correlation between proprioception error and FRT were not found in this study, which may be due to different assessing methods.
Worse performance of TUG in participants with type 2 DM compared with non-DM individuals, has been reported in previous studies [23,24]. TUG performance requires various mobility skills of sit-to-stand, straightahead gait and turning, and uses more information processing than other balance tests [25]. Sit to stand time and temporospatial gait parameters were declined in older adults with type 2 DM, compared with the non-DM group [26,27]. In this study, a higher number of sensory impairments were associated with poorer balance control, reflected in TUG performance. Participants with type 2 DM with all three sensory impairments, also demonstrated worse performance, compared to the groups without sensory impairment. Interestingly, older adults with type 2 DM although not presenting sensory impairment, also had poorer performance of TUG, compared with older adults without DM. This implies that subclinical changes of sensory functions may play a critical role in balance control [6].
Declined strength of knee and ankle muscles has been reported in persons with type 2 DM, compared with the non-DM group [26,28-30]. Worse muscle quality associated with type 2 DM, including low muscle density and muscle mass in people with type 2 DM, have been documented [26,28-30]. Muscle decline in type 2 DM were associated with increased levels of inflammatory cytokines, i.e., interleukin-6, and tumor necrosis factor-alpha [29]. Poor glycemic control and duration of DM exposure, also reportedly affected muscle function [31]. However, HbA1c and duration of DM exposure were not different, among groups with type 2 DM in this study.
This study found reduced muscle strength in the distal part of lower limbs, i.e., plantarflexors and dorsiflexors in the groups with less number of sensory impairments. The deterioration process of motor nerve fibers that leads to muscle weakness and atrophy is known to be length dependent [32]. Muscle loss usually occurs initially in the distal part, i.e., the feet, and progress into the lower limb. The type 2 DM group without sensory impairments also had less strength of ankle plantarflexor, compared to the non-DM group. The possible explanation is the neuropathic process of DPN, which predominantly involves motor nerve fibers in the early stage, thus presenting as weakened muscles with no sensory symptoms [10]. Severity of DPN was suggested to be associated with muscle strength. However, the relationship was not found with other DM complications, such as diabetic retinopathy and nephropathy [10].
Association between sensory impairment, balance performance, and lower limb muscle strength were observed in this study. The number of sensory impairments and lower limb muscle strength, also influenced the performance of balance tests. In the final regression model, type 2 DM with two and three sensory impairments were contributing factors of FRT with 11% variance accounted. Type 2 DM with at least two sensory impairments, denotes reduction of approximately two inches in FRT.
The combination of three sensory impairments in older adults with type 2 DM, ankle dorsiflexors muscle strength and age were presented as contributing factors of TUG with 24% variance accounted in the final model. Having type 2 DM with three sensory impairments denotes an increment of approximately 5 seconds in TUG. One year of advancing age, could predict 0.2 seconds increment of TUG. One kilogram increase of the ankle dorsiflexors strength, predicted 0.4 seconds reduction of TUG. Age has been demonstrated as a significant factor for TUG in older adults with type 2 DM [33]. Increased time to complete TUG gradually progressed as age increased [33]. Aligned with previous studies [4,11,24,34], impairments of all three sensory systems in older adults with type 2 DM, were the crucial factor of poorer balance. Ankle dorsiflexors strength also contributed to results of TUG, as it played a key role in the gate cycle. During heel strike, ankle dorsiflexors eccentrically contact to control rotation of the foot onto the ground, and prevent the foot from slapping the ground. The strength of ankle dorsiflexors in older adults was correlated with functional base of support, and threshold perturbation acceleration required for heel-rise that could limit restoration of balance, while standing on toes [35]. Too, ankle dorsiflexors strength was reported as a predictor of falling among older people with 17% variance accounted [36].
Functional balance performance assessed by RT was not different among groups in this study. RT mainly assesses proprioception [37]. So, participants could rely on somatosensory and vestibular inputs during the test [18]. We also found that lower limb proprioception was not different among groups. Although greater proprioception error was hypothesized especially for participants with DPN results were not aligned with the hypothesis. Similar balance performance identified by RT in type 2 DM groups with different patterns of sensory impairment may be a result of compensatory process among sensory functions. Sensory systems may be able to compensate each other in case another system was impaired. There were previous reports showing high percentage of normal proprioceptive sensitivity in older adults with type 2 DM [38,39]. Incidences of decline in deep tendon reflex (83%), touch sensitivity (31%), and vibration perception (15%) were noted as more obvious than deterioration of sensitivity of joint position sense (7%) in older adults with neuropathy [39].
Limitations
This study had some limitations. There were small samples in some groups of sensory impairment pattern, which may affect power of analysis. Overall results suggest that the sensory system may compensate each other in case some impairments are presented. However, the mechanism of compensation such as ability to adjust processing of sensory information, could not be explained by results of this study. Other factors of balance control such as cognitive impairment, depression, fear of falling, or body mass index, may also influence the effectiveness of this compensation mechanism, and balance performance. So, these factors should be considered in future studies.
Clinical implications
Poorer balance performance was observed in older adults with type 2 DM, and worsened muscle strength was found with higher numbers of sensory impairments. This finding implies that balance impairments should be focused on in older adults with type 2 DM, even ones without sensory impairments. DM complications of DPN, visual function, and vestibular function should be monitored. Interventions for preventing or rehabilitating sensory functions are necessary for fall prevention in this population. Also, strengthening exercises of lower limb muscles, especially ankle dorsiflexors, are recommended in older adults with type 2 DM, including ones without sensory impairments for preventing progressive complications and maintaining balance performance.
Conclusion
This study showed a variety of sensory impairment patterns and reduced muscle strength in older adults with type 2 DM. Greater number of sensory impairments accompanied with reduced lower limb muscle strength and advanced age contributed to worse balance performance in older adults with type 2 DM.
Notes
No potential conflict of interest relevant to this article was reported.
Conceptualization: Kraiwong R, Vongsirinavarat M. Methodology: Kraiwong R, Vongsirinavarat M, Hiengkaew V, von Heideken Wågert P. Formal analysis: Kraiwong R, Vongsirinavarat M. Project administration: Krai-wong R. Visualization: Kraiwong R, Vongsirinavarat M. Writing – original draft: Kraiwong R, Vongsirinavarat M. Writing – review and editing: Vongsirinavarat M, Hiengkaew V, von Heideken Wågert P. Approval of final manuscript: all authors.
Acknowledgements
We want to thank all participants and personnel from the clinical settings used for data collection.