Clinical, Electrophysiological Findings in Adult Patients with Non-traumatic Plexopathies
Article information
Abstract
Objective
To ascertain the etiology of non-traumatic plexopathy and clarify the clinical, electrophysiological characteristics according to its etiology.
Method
We performed a retrospective analysis of 63 non-traumatic plexopathy patients that had been diagnosed by nerve conduction studies (NCS) and needle electromyography (EMG). Clinical, electrophysiological, imaging findings were obtained from medical records.
Results
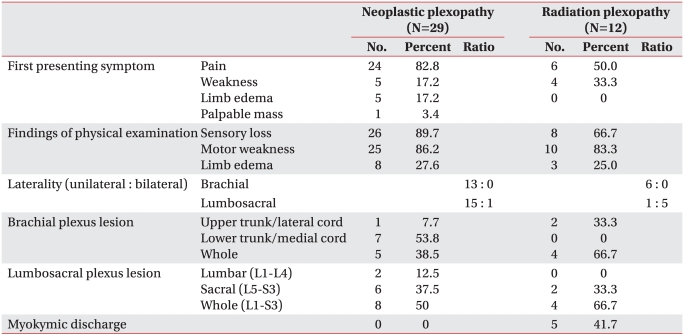
We identified 36 cases with brachial plexopathy (BP) and 27 cases with lumbosacral plexopathy (LSP). The causes of plexopathy were neoplastic (36.1%), thoracic outlet syndrome (TOS) (25.0%), radiation induced (16.7%), neuralgic amyotrophy (8.3%), perioperative (5.6%), unknown (8.3%) in BP, while neoplastic (59.3%), radiation induced (22.2%), neuralgic amyotrophy (7.4%), psoas muscle abscess (3.7%), and unknown (7.4%) in LSP. In neoplastic plexopathy, pain presented as the first symptom in most patients (82.8%), with the lower trunk of the brachial plexus predominantly involved. In radiation induced plexopathy (RIP), pain was a common initial symptom, but the proportion was smaller (50%), and predominant involvements of bilateral lumbosacral plexus and whole trunk of brachial or lumbosacral plexus were characteristic. Myokymic discharges were noted in 41.7% patients with RIP. Abnormal NCS finding in the medial antebrachial cutaneous nerve was the most sensitive to diagnose TOS. Neuralgic amyotrophy of the brachial plexus showed upper trunk involvement in all cases.
Conclusion
By integrating anatomic, pathophysiologic knowledge with detailed clinical assessment and the results of ancillary studies, physicians can make an accurate diagnosis and prognosis.
INTRODUCTION
Common conditions of the peripheral nervous system disorders are entrapment neuropathy, radiculopathy, and peripheral polyneuropathy. However, brachial plexopathy (BP) and lumbosacral plexopathy (LSP), are relatively uncommon.1 BP accounts for approximately 14% of all isolated upper limb neurologic lesions,2 and LSP accounts for a smaller percent of isolated lower limb neurologic lesions.
The nerve fibers passing through the plexus can be injured by various mechanisms such as laceration, compression, traction, stretch, radiation, and ischemia.3 By the nature of plexus injury, traumatic plexopathy is the most common, and there are many other causes such as neoplastic, radiation induced, perioperative, neuralgic amyotrophy, thoracic outlet syndrome (TOS), infectious, and diabetic conditions.3,4 In case of negative history of trauma, it is important to clarify the underlying etiology since significant differences occur in diagnostic approach, treatment plan, and prognosis.
We identified the causes of non-traumatic plexopathy in adult patients, and clarified the clinical, electrophysiological characteristics, and imaging findings according to its etiology.
MATERIALS AND METHODS
We analyzed the medical records, electrophysiological, and imaging findings of patients with non-traumatic plexopathy who were 20 years old or above and who had undergone nerve conduction studies (NCS) and needle electromyography (EMG) at our electrophysiological laboratory between January 1999 and January 2010. The diagnosis of plexopathy was confirmed using the following criteria: 1) abnormal NCS findings conforming to the anatomical distribution of the plexus when compared with our normative values, or a difference more than 50% in amplitude of nerve action potentials between the symptomatic and asymptomatic side when normative values were not available in certain nerves, such as musculocutaneous, axillary, suprascapular, radial, superficial radial, femoral, lateral femoral cutaneous, and saphenous nerve, and 2) evidence of denervation potentials or neuropathic potentials conforming to the anatomical distribution of the plexus in needle EMG, and 3) no evidence of denervation potentials in paraspinal muscles, and 4) no evidence of cervical or lumbosacral radiculopathy in magnetic resonance imaging (MRI) of the spine.
We excluded cases where a clear history of trauma was directly related to plexus injury, such as traffic accident, falls, and associated with medical procedure or surgery, and we classified plexopathy based on the nature of injury.3,4
The causes of non-traumatic plexopathy were defined as follows. In patients with a history of cancer, the diagnosis of neoplastic plexopathy was confirmed by imaging findings, which showed direct invasion of the plexus by cancer or compressive lesion by metastatic enlarged lymph nodes or soft tissues. In patients without a history of cancer, the diagnosis of neoplastic plexopathy was made by confirming histopathologic findings. Radiation induced plexopathy (RIP) was defined as cases with a history of radiation therapy, but without evidence of recurrent tumor or metastatic invasion by imaging findings. TOS was defined as follows: 1) sensory symptoms in the medial forearm and fourth-fifth finger; 2) objective sign of sensory loss in medial antebrachial cutaneous (MABC) or ulnar nerve areas; 3) electrophysiological findings corresponding to lower trunk lesion, but no evidence of carpal tunnel syndrome, ulnar neuropathy, or cervical radiculopathy, and 4) Imaging findings suggesting the diagnosis of TOS: presence of either elongated transverse process or cervical rib on plain radiography, or evidence of compression of neurovascular bundle at thoracic outlet on either computed tomography (CT) angiography or brachial plexus MRI. Neuralgic amyotrophy was defined as cases that had a history of sudden onset of severe pain at unilateral upper or lower limb, followed by weakness and/or muscle atrophy with the exclusion of other possible causes of plexopathy. We excluded cases showing a pattern of mononeuropathy, such as long thoracic mononeuropathy, or suprascapular mononeuropathy on electrophysiological findings, and thus, we included cases which showed a plexopathy pattern. Perioperative plexopathy was defined as cases with an onset after surgery, but without evidence of direct trauma though the surgical procedure, as well as exposure of the plexus to the surgical field.
RESULTS
Thirty six patients (mean age, 49.3±11.9 years; male to female ratio, 14 : 22) with BP, and 27 patients (mean age, 52.7±13.7 years; male to female ratio, 6 : 21) with LSP were identified. The causes of BP included 13 patients (36.1%) with neoplastic plexopathy, 9 patients (25.0%) with TOS, 6 patients (16.7%) with RIP, 3 patients (8.3%) with neuralgic amyotrohy, 2 patients (5.6%) with perioperative, and 3 patients (8.3%) of unknown cause. The causes of LSP included 16 patients (59.3%) with neoplastic plexopathy, 6 patients (22.2%) with RIP, 2 patients (7.4%) with neuralgic amyotrophy, one patient (3.7%) with psoas muscle abscess, 2 patients (7.4%) with unknown cause (Table 1).
Brachial plexus lesions
Neoplastic brachial plexopathy (N=13)
There were 5 males and 8 females with the mean age of 52.9±11.3 years. Of the 7 patients (53.8%) who had a previous diagnosis of cancer, 5 were undergoing cancer therapy, and 2 had recurrent after cancer therapy. Six patients (56.2%) who had no history of cancer, encountered the diagnosis of primary cancer due to neoplastic BP. Secondary plexus tumors accounted for 84.6% (n=11) of neoplastic BP, composed of 3 breast cancers, 3 lung cancers, 2 diffuse large B cell lymphomas, one thyroid cancer, one desmoid tumor, and one chloroma (extramedullary myeloid tumor). Two cases (15.4%) were due to primary brachial plexus tumor, one schwannoma and one malignant peripheral nerve sheath tumor (Table 2) (Fig. 1-A).
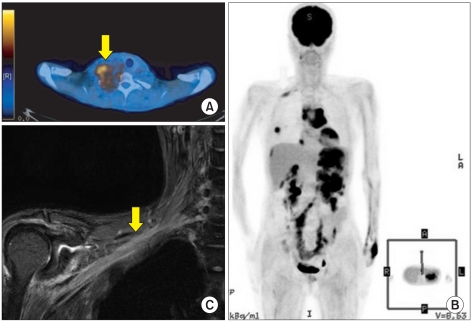
(A) PET-CT in a patient with malignant peripheral nerve sheath tumor (arrow) in right brachial plexus. Histopathologic confirmation was malignant transformation originating from neurofibroma. (B, C) Brachial plexus MRI and PETCT in a patient with chloroma. Brachial plexus MRI shows diffuse enhancement and swelling (arrow) in brachial plexus from trunk to cord level which suggests peri-plexus infiltration of chloroma.
Initial clinical manifestations were pain (n=9), pain and weakness (n=1), weakness (n=1), and palpable neck mass (n=1). Physical examination revealed sensory loss (n=11), weakness (n=11), Horner syndrome (n=3), and upper limb edema (n=1).
NCS and needle EMG demonstrated that all patients had a unilateral lesion, and frequency of location was higher in lower trunk/medial cord lesion (n=7) and whole plexus lesion (n=5) than in upper trunk/lateral cord lesion (n=1). Based on imaging findings, supraclavicular, infraclavicular, and whole plexus lesions were noted in 3, 5, and 5 cases respectively. One case with acute myeloid leukemia did not show compressive lesion but direct perineural spread (Fig. 1-B, C).
Radiation induced brachial plexopathy (N=6)
There were one male and 5 females with the mean age of 45.2±9.2 years. Underlying tumors included 2 breast cancers, 2 nasopharyngeal cancers, one thyroid cancer, and one fibromatosis. Mean interval between radiation and onset of symptom was 2.9±3.7 years (median interval, 4.25 years; range, 3 months to 7 years).
Initial clinical manifestations were weakness (n=2), weakness and pain (n=1), pain (n=1), numbness (n=1), and limitation of shoulder range of motion (n=1). Physical examination revealed sensory loss (n=4), weakness (n=4), upper limb edema (n=1), and no Horner syndrome.
NCS and needle EMG demonstrated that all patients had a unilateral lesion. The most common location was whole plexus lesion (n=4), followed by upper trunk/lateral cord lesion (n=2), and there was no lower trunk/medial cord lesion (Table 3). Myokymic discharges which suggest RIP were detected in 2 patients (33.3%). Brachial plexus MRI of one patient demonstrated the fibrosis of tissues around the plexus (Fig. 2-A, B).
Comparison of Clinical, Electrophysiological Characteristics between Neoplastic and Radiation Induced Plexopathy (N=41)
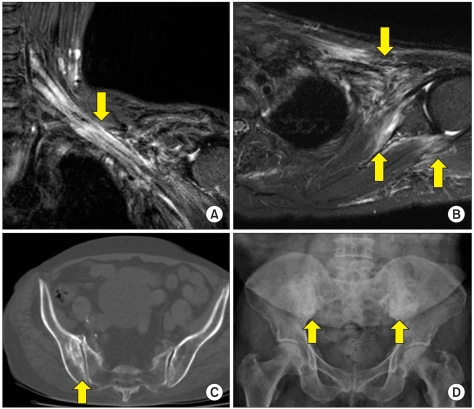
Brachial plexus MRI in a patient with radiation induced brachial plexopathy (A, B). Images show signal change and enhancement in brachial plexus [arrow, A], and fibrosis in subscapularis, teres minor, pectoralis, and infraclavicular soft tissue [arrow, B] which suggest radiation induced fibrosis. (C) Pelvis CT demonstrates osteosclerotic cystic lesions (arrow) in right ilium which suggest association with radiation. (D) Pelvis X ray shows dense radiopacity lesions (arrow) in both ilia which suggest osteonecrosis.
Thoracic outlet syndrome (N=9)
7 patients were females with the mean age of 48.7±16.5 years. All patients had a unilateral lesion. Physical examination showed thenar atrophy in 6 patients, and muscle weakness in 8 patients. All patients showed grade 4-4+ of abductor pollicis brevis on Medical Research Council scale, and 2 patients showed grade 4+ of abductor digiti minimi and first dorsal interossei. Positive finding was noted in 3 patients with Adson test, 2 with Wright test, one with 90 degree abduction-external rotation test, and one with upper limb tension test.
Of 8 patients (88.9%) who showed abnormal findings on plain radiography, 5 had elongated transverse process, 2 had cervical rib, and one had both. Of 3 patients who underwent Brachial plexus MRI, one had an evidence of compression of neurovascular bundle. Electrophysiological findings were consistent with a lesion affecting lower trunk of brachial plexus. NCS were conducted in 7 patients of MABC nerves, and in all patients of median and ulnar nerves. Abnormal findings were noted in MABC sensory nerve action potential (SNAP), ulnar SNAP, median compound muscle action potential (CMAP), and ulnar CMAP: 85.7%, 77.8%, 55.6%, and 33.3% respectively. Denervation potentials on needle EMG were noted in 8 patients. Muscles observed denervation potentials included abductor pollicis brevis (n=5), abductor digiti minimi (n=3), first dorsal interossei (n=3), flexor digitorum profundus (n=1), and extensor indicis proprius (n=1).
Neuralgic amyotrophy of brachial plexus (N=3)
There were 2 males and one female. Age was 34, 49, and 60 years respectively. 2 patients showed left side involvement and one right side involvement. Interval between onset of pain and development of weakness was 1, 2, and 7 days respectively. All patients showed grade 1-2 of shoulder flexors and abductors, and grade 3-4 of elbow flexors. Elbow extensors, wrist extensors, wrist flexors, hand intrinsic muscles showed normal grade. Sensory tests were normal and Spurling sign was negative.
Abnormal NCS findings were noted in axillary nerve (n=3), musculocutaneous nerve (n=2), suprascapular nerve (n=1), and lateral antebrachial cutaneous nerve (n=1). Denervation potentials were detected in muscles innervated by suprascapular (n=2), musculocutaneous (n=2), and axillary nerve (n=2). Neuropathic potentials were detected in muscles innervated by suprascapular (n=1), musculocutaneous (n=1). Electrophysiological findings were consistent with a lesion affecting upper trunk of brachial plexus.
Perioperative brachial plexopathy (N=2)
There were one case with median sternotomy and one case with subtotal gastrectomy. Patient with median sternotomy showed grade 1 of hand intrinsic muscles while normal grade of shoulder, elbow, wrist joint muscles. Electrophysiological findings were compatible with lower trunk lesion.
Patient with subtotal gastrectomy showed grade 4 of shoulder abductors and elbow flexors. Electrophysiological findings were compatible with upper trunk lesion.
Lumbosacral plexus lesions
Neoplastic lumboscral plexopathy (N=16)
There were 3 males and 13 females with the mean age of 51.7±13.9 years. Predominance of female was observed. 13 patients (81.3%) had a previous diagnosis of cancer, 3 patients (18.7%) who had no history of cancer, were diagnosed with underlying primary tumor due to neoplastic LSP. Secondary plexus tumors accounted for 93.8% (n=15) of neoplastic LSP, while primary plexus tumors accounted for 6.2% (n=1). Secondary plexus tumors included 7 uterine cervix cancers (43.8%), 3 sarcomas, 2 colorectal cancers, one bladder cancer, one vulvar cancer, and one nasopharyngeal cancer (Table 2).
Initial clinical manifestations were pain (n=11), pain and limb edema (n=2), pain and weakness (n=1), and weakness and limb edema (n=2). Pain was the most predominant initial symptom as in neoplastic BP. Physical examination revealed sensory loss (n=15), weakness (n=14), and lower limb edema (n=7).
NCS and needle EMG demonstrated that 15 patients (93.8%) had a unilateral lesion, one patient (6.2%) with uterine cervix cancer had a bilateral lesion. The most common location was whole plexus (L1-S3) lesion (n=8), followed by sacral plexus (L5-S3) lesion (n=6), and lumbar plexus (L1-L4) lesion (n=2).
Radiation induced lumbosacral plexopathy (N=6)
All patients were females with the mean age of 61.0±9.1 years. Underlying tumors included 5 uterine cervix cancers, and one colorectal cancer. Mean interval between radiation and onset of symptom was 7.8±8.8 years (median interval, 6.5 years; range, 3 months to 24 years). Initial clinical manifestations were pain (n=4), paresthesia (n=1), and weakness (n=1). Physical examination revealed weakness (n=6), sensory loss (n=4), and lower limb edema (n=2).
NCS and needle EMG demonstrated that 5 patients (83.3%) had a bilateral lesion, and one patient (16.7%) had a unilateral lesion. The most common location was whole plexus lesion (n=4), followed by sacral plexus lesion (n=2), and there was no lumbar plexus lesion (Table 3). Myokymic discharges were detected in 3 patients (50%). Two patients showed osteonecrosis in illium by pelvis X-ray and CT (Fig. 2-C, D), which suggested association with RIP.
Neuralgic amyotrophy of lumbosacral plexus (N=2)
Both patients showed unilateral involvement, one with left side and one with right side. The interval between onset of pain and development of weakness was several hours and 2 weeks respectively. Physical examination revealed sensory loss in one patient, and weakness in 2 patients. A 37-year-old man showed grade 4+ of muscles innervated by lumbar plexus, and a 53-year-old man showed grade 3-4 of muscles innervated by lumbar plexus and lumbosacral trunk (L2-L5). Electrophysiological findings were consistent with lumbar plexus lesion in a 37-year-old man, and lumbosacral plexus lesion 53-year-old man.
DISCUSSION
Neoplastic plexopathy can be divided into primary or secondary. While primary plexus tumors are rare and usually benign, secondary plexus tumors are the most common and all types are malignant. Secondary plexus tumors involve the plexus by means of extrinsic compression or infiltration from adjacent structures or spread from distant metastasis.5,6
In this study, there were two cases of primary malignant tumors. The histopathology revealed that both cases had undergone malignant transformation from plexiform neurofibroma in patients with neurofibromatosis type 1 (NF1). Most primary malignant tumors originate from plexiform neurofibroma,7 and approximately 5-10% of patients with NF1 develop malignant change, usually in a preexisting plexiform neurofibroma.8 Secondary plexus tumors develop late in the course of the disease.9 The most common tumors reported are lung cancer, breast cancer, and lymphoma in BP,10 and colorectal cancer, sarcoma, uterine cervix cancer, and lymphoma in LSP.11 Our results showed that the percent of uterine cervical cancer was higher than previously reported. The high prevalence of uterine cervical cancer in Korea may contribute to this finding.
It is known that neoplastic plexopathy generally begins with pain, followed by sensory loss and weakness, and that pain is the most common presenting symptom (75-98%);10-13 our results were similar to these clinical findings (Table 3). Limb edema was relatively infrequent in BP, (7.7%) but was more commonly seen in LSP (43.8%), and this finding was similar to previous studies.10,11,13 For the purpose of comparing the clinical differences between neoplastic plexopathy and RIP, we described BP and LSP together (Table 3). Brachial and lumbosacral plexus, however, are distinctly different anatomical structures. The brachial plexus, originating from ventral primary rami of the lower 4 cervical and the first thoracic roots (C5-T1), is the most complex structure, and supplies most of the upper extremity and shoulder.6 The lumbosacral plexus is composed of 2 plexus, lumbar and sacral plexus, originating from ventral primary rami of the 5 lumbar and the first 3 sacral roots (L1-5 and S1-3), and supplies the lower extremity.4
Generally, plexopathies are classified according to the region involved, such as supraclavicular, infraclavicular, and whole plexus lesions. The supraclavicular plexus is further divided into upper (C5 and C6 roots and upper trunk), middle (C7 root and middle trunk), and lower (C8 and T1 roots and lower trunk) portions, and the infraclavicular plexus is further divided into 3 cords (medial, lateral, and posterior cords), and their terminal branches.6 This classification system, however, was not used in this study. In some cases, it was difficult to localize the lesion as NCS and needle EMG had not been performed sufficiently. This is a limitation of retrospective study. In another cases, it was also difficult to localize the lesion, although NCS and needle EMG had been performed sufficiently. This is a limitation of NCS and needle EMG. We classified the plexus lesions in 3 groups, upper trunk/lateral cord, lower trunk/medial cord, and whole plexus lesion based on electrophysiological findings. According to this classification, different patterns of involvement were noted among underlying etiologies, so it is possible to determine their clinical characteristics.
Our results revealed that frequency of location of neoplastic BP was higher in lower trunk/medial cord and whole plexus lesion and that of neoplastic LSP was higher in sacral plexus lesion and bilateral lesion. This finding suggests that invasion of the brachial plexus is characterized by superior extension of lung cancer ("Pancoast" syndrome or superior sulcus tumor) or breast cancer,14 and that of the lumbosacral plexus by direct local extension of uterine cervical cancer or colorectal cancer within the pelvis.11,12
RIP typically presents as a painless lesion with paresthesia and numbness, and lesion of the lumbosacral plexus is associated with limb edema and bilateral involvement.13,15-17 In contrast to previous reports, our results showed that the rate of pain presenting with the first symptom was 50% (Table 3), which was higher than previous reports, and the development of limb edema was not significantly different between RIP and neoplastic plexopathy. The small sample size and the limitations of a retrospective study may contribute to these results. Further study with a large sample size is needed for more conclusive results. Myokymic discharges were detected in 5 of 12 patients (41.7%) with RIP. Several studies reported that approximately 60% of patients with RIP show myokymic discharges on needle EMG,18,19 which is quite unusual with neoplastic plexopathy.
True neurogenic TOS demonstrates physical and electrophysiological findings consistent with a lesion affecting the C8/T1 roots or lower trunk of brachial plexus.20 Provocation maneuvers, such as Adson test, Wright test, 90-degree abduction-external rotation test, and upper limb tension test, are implemented to diagnose TOS, although they have a low specificity with a false-positive rate of 11% in Adson test, 62% in 90-degree abduction-external rotation test.21 NCS typically demonstrate reduced amplitude in median CMAP and ulnar SNAP, reduced or normal amplitude in ulnar CMAP, and normal median SNAP. Recent studies reported that reduced amplitude in MABC SNAP was the most sensitive test to diagnose TOS.22-25 Our results were consistent with these findings, therefore NCS of MABC nerve should be conducted when neurogenic TOS was suspected.
Classic postoperative paralysis, considered to develop due to traction and compression by surgical position or supporting brace, has a full recovery course by 3-4 months.6,26 Etiology of post-median sternotomy BP, commonly accepted, is a traction or compression secondary to rotation of the first rib with excessive retraction of the rib cage.20,27 The distinction between TOS and post-median sternotomy is needed since they present symptoms and signs of C8/T1 roots. Patients with post-median sternotomy have C8 fibers preferentially affected at the root level while patients with TOS have T1 fibers disrupted at the upper trunk level. In contrast to TOS, post-median sternotomy demonstrate that the MABC SNAP and median CMAP are less severely affected than the ulnar SNAP and CMAP.
Neuralgic amyotrophy is characterized by acute onset of severe pain associated with multifocal muscle weakness and subsequent atrophy that usually appears as the pain lessens.28 Although the most common, typical phenotype of an attack involves the upper and middle trunk of the brachial plexus, including long thoracic nerve, some cases involve the lumbosacral plexus and lower cranial nerves.28-32 It is likely that, rather than a single disease entity, neuralgic amyotrophy is a syndrome comprising different underlying mechanisms, phenotypes and prognoses.33
The limitations of our study include that the small sample size and that it was conducted retrospectively. In order to clarify etiology and frequency of non-traumatic plexopathy, and to compare with previous reports, more and larger sized multicenter study may be necessary.
CONCLUSION
The most common etiology of non-traumatic plexopathy in adult patients was neoplastic plexopathy, which almost always originated from secondary plexus tumors. Since the rate of history of cancer was low in patients with neoplastic BP, we should not overlook the possibility of neoplasm when BP is suspected.
Clinical distinctions of neoplastic plexopathy from RIP were that most patients with neoplastic plexopathy presented with pain as the first symptom, and the proportion of patients with pain as the first symptom was higher than in patients with RIP. Neoplastic plexopathy showed unilateral lumbosacral lesion, while RIP showed bilateral lumbosacral lesion. Electrophysiological findings demonstrated that lower trunk/medial cord lesion was predominantly involved in neoplastic BP, while whole plexus lesion was more involved in radiation induced BP. TOS involves C8 and T1 roots or lower trunk of brachial plexus, but it preferentially affects T1 fibers. MABC SNAP was the most sensitive diagnose TOS.
By the acquisition of clinical and electrophysiological findings according to etiology, physicians can make an accurate diagnosis and prognosis.