- Search
| Ann Rehabil Med > Volume 35(6); 2011 > Article |
Abstract
Objective
To investigate the effect of repetitive transcranial magnetic stimulation (rTMS) on recovery of the swallowing function in patients with a brain injury.
Method
Patients with a brain injury and dysphagia were enrolled. Patients were randomly assigned to sham, and low and high frequency stimulation groups. We performed rTMS at 100% of motor evoked potential (MEP) threshold and a 5 Hz frequency for 10 seconds and then repeated this every minute in the high frequency group. In the low frequency group, magnetic stimulation was conducted at 100% of MEP threshold and a 1 Hz frequency. The sham group was treated using the same parameters as the high frequency group, but the coil was rotated 90В° to create a stimulus noise. The treatment period was 2 weeks (5 days per week, 20 minutes per session). We evaluated the Functional Dysphagia Scale (FDS) and the Penetration Aspiration Scale (PAS) with a videofluoroscopic swallowing study before and after rTMS.
Results
Thirty patients were enrolled, and mean patient age was 68.2 years. FDS and PAS scores improved significantly in the low frequency group after rTMS, and American Speech-Language Hearing Association National Outcomes Measurements System Swallowing Scale scores improved in the sham and low frequency groups. FDS and PAS scores improved significantly in the low frequency group compared to those in the other groups.
Dysphagia is associated with high levels of morbidity, mortality, and financial costs. Lindgren and Janzon reported that the prevalence of dysphagia among patients >50 years was 16-22%.1 Furthermore, specific populations, such as those with head injuries, patients who have had cerebrovascular accidents, or those with Parkinson's disease, show a 20-40% rate of oropharyngeal dysphagia.2 Dysphagia develops after acute brain injury, but most patients recover their swallowing function within a few weeks. The extent of recovery varies widely from patient to patient. The prevalence of dysphagia is higher and symptom duration is longer after a brain stem injury than after other types of brain injury.3 However, some reports indicate that 40-50% of patients with an acute brain injury and lesions in regions other than the brain stem experience dysphagia.4,5 Thus, immediate and intensive treatment is needed to prevent complications caused by dysphagia.
Several methods have been used to treat dysphagia, including oral and facial sensory training, oral and pharyngeal muscle strengthening, compensatory techniques, prosthetic devices, and surgery.6 Electrical stimulation has recently emerged as a new treatment modality. However, the validity and mechanism of these procedures has not been adequately documented.7-9 Although many treatment options are available, many patients continue to suffer from dysphagia; thus, new treatment strategies are urgently required.
Noninvasive brain stimulation techniques including repetitive transcranial magnetic stimulation (rTMS) and transcranial direct current stimulation have become available. rTMS modulates cortical excitability by focally stimulating the cortical region.10 Hummel et al. found that an interhemispheric imbalance is evident in patients with brain injury, and that use of noninvasive brain stimulation techniques diminish this imbalance.11,12 It is known that high-frequency stimulation of an affected hemisphere increases cortical excitability, whereas low-frequency stimulation of the unaffected hemisphere lowers excitability. Several studies on motor function recovery after noninvasive brain stimulation have been reported,10-15 and several studies have found that working memory improves after such stimulation.16,17
However, only a few studies have examined the utility of rTMS in terms of recovery of the swallowing function. Jefferson et al. reported that rTMS at a frequency of 5 Hz increases excitability of the contralateral cortex, resulting in improvement in pharyngeal muscle function.18 Verin and Leroi found that swallowing function improved significantly in seven patients who suffered from stroke after 5 days of treatment of the unaffected hemisphere with 1 Hz-rTMS.19 Khedr et al. reported that the swallowing function in patients with a brain injury improved significantly compared to that in a sham-stimulated group after applying 3 Hz-rTMS to the affected hemispheres on each of 5 consecutive days.3 These studies clearly show that no standard rTMS protocol for treating dysphagia has been developed, and no prior report has compared the effects of high- and low-frequency stimulation to this end.
Thus, we investigated the effect of rTMS on dysphagia in patients with a brain injury by comparing high-frequency, low-frequency, and sham rTMS stimulation.
Patients with dysphagia that occurred after an acute brain injury and whose symptom onset occurred after <3 months were enrolled. All patients had unilateral hemispheric brain lesions, but none had lesions on the brain stem or cerebellum. Patients were randomly assigned to high-frequency, low-frequency, and sham stimulation groups. All patients fulfilled the following inclusion criteria: the presence of dysphagia after acute brain injury, and disease that was localized to a unilateral cerebral hemisphere (as documented by computerized tomography or magnetic resonance imaging). Exclusion criteria included a prior diagnosis of another neurological disease, an unstable medical condition, severe cognitive impairment, severe aphasia, and/or a history of seizure. Patients who had difficulty swallowing food, or who coughed during or after swallowing, or in whom food materials were found in the oral cavity or pharynx, were defined as suffering from dysphagia. Among such patients, those who showed penetration or aspiration on a videofluoroscopic swallowing study (VFSS) were enrolled. Written informed consent was obtained from all subjects prior to inclusion; this study was approved by the Asan Medical Center Hospital Ethics Committee and performed in accordance with the Declaration of Helsinki.
Before performing rTMS, we evaluated motor-evoked potentials (MEPs) of the bilateral mylohyoid muscles using a Magstim 200В® nerve stimulator (Magstim Co, Dyfed, UK). After tying a linen cap tightly to the head of the patient, magnetic stimulation using a circular coil (external diameter, 9 cm) was performed over the bilateral anterolateral scalp, commencing at the vertex. The mylohyoid muscle on the hemiplegic side was the target muscle. The motor threshold (MT) was defined as the minimal stimulus intensity required to produce MEP of >100 ВөV peak-to-peak amplitude in three of five consecutive trials on the mylohyoid muscles. The location yielding the largest response amplitude was termed the "hot spot", and we delivered magnetic stimulation to that point.
The Magstim instrument was used to deliver stimulatory trains using a figure-eight coil cooled with air. rTMS was performed on the ipsilesional hemisphere hot spot at 100% of each MEP threshold, at 5 Hz, for 10 sec, and repeated every minute for 20 minutes in the high-frequency stimulation group (total, 1,000 pulses). For low-frequency stimulation, a 1 Hz stimulation at 100% MT was delivered for 20 minutes (total, 1,200 pulses) on the contralesional hemisphere hot spot. Sham stimulation was applied using the protocol employed for high-frequency stimulation, but the coil was rotated through 90В°; thus, no stimulation was applied but the noise that was characteristic of stimulation was present. During rTMS, all patients wore ear plugs to prevent hearing damage. rTMS was performed once per day for 20 minutes on 10 consecutive days (five times per week for 2 weeks), and all patients received swallowing training comprised of oral and facial sensory training, oral and pharyngeal muscle training, compensatory techniques, and neuromuscular electrical stimulation on pharyngeal muscles during rTMS.
Modified Barthel Index values were recorded prior to applying rTMS to compare patient functional abilities. Both before and after rTMS, we evaluated the Functional Dysphagia Scale (FDS), the Penetration Aspiration Scale (PAS) using VFSS, and the American Speech-Language Hearing Association National Outcomes Measurements System Swallowing Scale (ASHA NOMS). FDS is a scale that was developed to quantify dysphagia severity,20 and PAS evaluates airway invasion.21 Both FDS and PAS can be evaluated with VFSS. ASHA NOMS describes the swallowing abilities of patients.22,23 Higher FDS and PAS scores indicate worse swallowing function, and higher ASHA NOMS scores indicate better swallowing function.
All statistical analyses were performed using SPSS version 18.0 (SPSS, Inc., Chicago, USA). Statistical analyses were performed using the Kruskal-Wallis test to compare baseline characteristics and FDS, PAS, and ASHA NOMS scores according to treatment (low-frequency, high-frequency, or sham) both before and after treatment. Paired comparisons within groups were analyzed using Wilcoxon's signed-rank tests. A p-value <0.05 was considered significant.
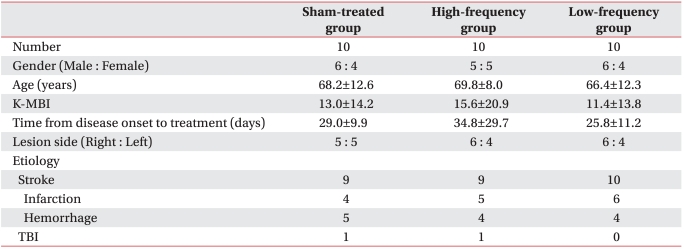
Thirty patients were enrolled, and their mean age was 68.2Вұ10.5 years. No patient experienced any adverse effects from the rTMS such as seizures or uncontrollable headaches. Patient demographic data are listed in Table 1; no significant difference was found among the three groups for demographics.
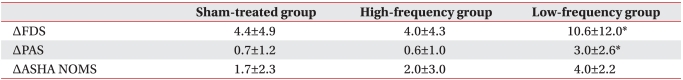
No significant difference in FDS, PAS, or ASHA NOMS scores was observed before treatment among the three groups. The ASHA NOMS score improved in both the sham and low-frequency stimulation groups, but the FDS and PAS scores improved after 10 consecutive rTMS sessions in only the low-frequency stimulation group (Fig. 1-A).
The changes in FDS and PAS values apparent after low-frequency stimulation were significantly higher than those seen after high-frequency or sham stimulation (Table 2).
The primary objective of this study was to investigate whether rTMS improves the swallowing function in patients with a brain injury. As a result, low-frequency rTMS improved FDS and PAS scores, whereas high-frequency and sham stimulation did not improve FDS and PAS scores. These results corresponded with previous studies.3,19,24 Hamdy et al. localized the swallowing function within the human motor cortex using TMS.25 The author reported that the swallowing musculature receives cortical projections from both hemispheres, but that a dominant hemisphere was evident. To date, several TMS studies seeking to improve swallowing function in patients with a brain injury have been reported, but they included patients with a brain stem stroke only, or were not randomized controlled trials.3,8,19 Thus, we investigated the effect of rTMS on the swallowing function in patients with a hemispheric brain injury.
We performed 10 consecutive rTMS sessions on dysphagic patients with a brain injury and found that rTMS delivered over the pharyngeal motor area improved dysphagic parameters compared to those in the sham-stimulation group; the effects were more marked when low-frequency stimulation was employed. Our results agree with those of previous studies. Khedr et al. found that applying rTMS improves the swallowing function in patients with either brain stem or monohemispheric strokes. The author performed five consecutive sessions using 3 Hz rTMS (delivering a total of 300 TMS pulses daily) and compared the results with those of a sham-stimulated group.3,24 A few reports on the effects of rTMS on dysphagia in patients with a hemispheric stroke have appeared, but the rTMS was delivered at a 3 Hz frequency. No prior study has compared the effects of high-and low-frequency stimulation with that of sham stimulation.
Applying high-frequency rTMS to the affected hemisphere enhances cortical excitability, whereas low-frequency rTMS applied to the non-affected hemisphere decreases such excitability. Interhemispheric inhibition occurs in patients with a unilateral hemispheric brain injury, and both high- and low-frequency rTMS reduces this inhibition by increasing the excitability of the affected hemisphere or by lowering the excitability of the non-affected hemisphere.
In this study, only low-frequency rTMS improved dysphagia in patients with a unihemispheric brain injury. Because the swallowing musculature is innervated from both hemispheres, many patients recover from dysphagia. However, many patients with a unilateral hemispheric brain injury suffer from long-term dysphagia. This can be explained by interhemispheric inhibition after brain injury. According to Hamdy et al., the "dominant" hemisphere exerts the principal effect on swallowing function.25 This hemisphere is independent of handedness and, when injured, causes more severe dysphagia. Thus, patients with a unilateral hemispheric stroke with lesions in the hemisphere dominant in terms of the swallowing function can suffer from severe dysphagia. These authors also reported that recovery from dysphagia after a unilateral hemispheric stroke depends on regulating the non-affected hemisphere to reduce interhemispheric inhibition. Verin and Leroi also explained that improved dysphagia after low-frequency rTMS on the unaffected hemisphere is due to a decrease in interhemispheric inhibition.19 But, high-frequency rTMS on an affected hemisphere to decrease interhemispheric inhibition by increasing excitability of the affected hemisphere does not improve swallowing function.
In our study, ASHA NOMS scores improved in the sham and low frequency groups after rTMS. But, the change in the score was not significant when we compared the results among the three groups. So, improved ASHA NOMS scores in the sham and low frequency groups can be explained by an effect of natural recovery, but not by rTMS.
There are some limitations in our work. First, our sample size was small. Second is the lack of long-term follow-up. Third, previous studies performed rTMS at 3 Hz, whereas we performed rTMS at 1 and 5 Hz. Thus, this could make it difficult to compare the results of our study with those of previous studies. Fourth, patient composition was not homogeneous, and we did not consider lesion size or lesion location in the hemispheres. The brain injury mechanism and the extent of recovery may be different in patients with stroke and traumatic brain injury. However, no significant difference was observed in the etiologies among the groups. We suspect that heterogeneity of etiologies would cause a minor effect on our results. Finally, we defined the MT as the minimal intensity to achieve an MEP of at least 100 ВөV as Kim et al.,26 and this was higher than that of most previous studies, which used 50 ВөV. We also used a circular coil to measure MEPs, and a figure-eight coil for rTMS. Because a figure-eight coil generates high stimulation intensity in the center of the coil than that of a circular coil, the rTMS stimulus intensity would be larger than that of MEP. This could cause adverse effects such as seizures or headache. However, no patients in our study complained of adverse effects during or after rTMS. Because of these limitations, the possibility that the results of our study are due to natural recovery and not due to the effect of rTMS cannot be ruled out.
We showed that low-frequency rTMS improved swallowing function in patients with a unilateral hemispheric brain injury. Thus, low-frequency rTMS may be a valuable treatment modality to improve the swallowing function in patients with a brain injury and dysphagia. Further studies with a larger sample size and more homogeneous subject composition are needed.
References
1. Lindgren S, Janzon L. Prevalence of swallowing complaints and clinical findings among 50-79-year-old men and women in an urban population. Dysphagia 1991;6:187-192. PMID: 1778094.


3. Khedr EM, Abo-Elfetoh N, Rothwell JC. Treatment of post-stroke dysphagia with repetitive transcranial magnetic stimulation. Acta Neurol Scand 2009;119:155-161. PMID: 18771521.


4. Dennis M. Dysphagia in acute stroke: A long-awaited trial. Lancet Neurol 2006;5:16-17. PMID: 16361015.


5. Barer DH. The natural history and functional consequences of dysphagia after hemispheric stroke. J Neurol Neurosurg Psychiatry 1989;52:236-241. PMID: 2564884.



6. Langmore SE, Miller RM. Behavioral treatment for adults with oropharyngeal dysphagia. Arch Phys Med Rehabil 1994;75:1154-1160. PMID: 7944924.


7. Permsirivanich W, Tipchatyotin S, Wongchai M, Leelamanit V, Setthawatcharawanich S, Sathirapanya P, Phabphal K, Juntawises U, Boonmeeprakob A. Comparing the effects of rehabilitation swallowing therapy vs. Neuromuscular electrical stimulation therapy among stroke patients with persistent pharyngeal dysphagia: A randomized controlled study. J Med Assoc Thai 2009;92:259-265. PMID: 19253803.

8. Bulow M, Speyer R, Baijens L, Woisard V, Ekberg O. Neuromuscular electrical stimulation (nmes) in stroke patients with oral and pharyngeal dysfunction. Dysphagia 2008;23:302-309. PMID: 18437464.


9. Clark H, Lazarus C, Arvedson J, Schooling T, Frymark T. Evidence-based systematic review: effects of neuromuscular electrical stimulation on swallowing and neural activation. Am J Speech Lang Pathol 2009;18:361-375. PMID: 19726568.


10. Alonso-Alonso M, Fregni F, Pascual-Leone A. Brain stimulation in poststroke rehabilitation. Cerebrovasc Dis 2007;24(Suppl 1): 157-166. PMID: 17971652.

11. Hummel F, Celnik P, Giraux P, Floel A, Wu WH, Gerloff C, Cohen LG. Effects of non-invasive cortical stimulation on skilled motor function in chronic stroke. Brain 2005;128:490-499. PMID: 15634731.


12. Hummel FC, Cohen LG. Non-invasive brain stimulation: A new strategy to improve neuro rehabilitation after stroke? Lancet Neurol 2006;5:708-712. PMID: 16857577.


13. Fregni F, Boggio PS, Mansur CG, Wagner T, Ferreira MJ, Lima MC, Rigonatti SP, Marcolin MA, Freedman SD, Nitsche MA, Pascual-Leone A. Transcranial direct current stimulation of the unaffected hemisphere in stroke patients. Neuroreport 2005;16:1551-1555. PMID: 16148743.


14. Jang SH, Ahn SH, Byun WM, Kim CS, Lee MY, Kwon YH. The effect of transcranial direct current stimulation on the cortical activation by motor task in the human brain: An fmri study. Neurosci Lett 2009;460:117-120. PMID: 19450657.


15. Profice P, Pilato F, Dileone M, Ranieri F, Capone F, Musumeci G, P AT, Di Lazzaro V. Use of transcranial magnetic stimulation of the brain in stroke rehabilitation. Expert Rev Neurother 2007;7:249-258. PMID: 17341173.


16. Fregni F, Boggio PS, Nitsche M, Bermpohl F, Antal A, Feredoes E, Marcolin MA, Rigonatti SP, Silva MT, Paulus W, Pascual-Leone A. Anodal transcranial direct current stimulation of prefrontal cortex enhances working memory. Exp Brain Res 2005;166:23-30. PMID: 15999258.


17. Jo JM, Kim YH, Ko MH, Ohn SH, Joen B, Lee KH. Enhancing the working memory of stroke patients using tdcs. Am J Phys Med Rehabil 2009;88:404-409. PMID: 19620953.


18. Jefferson S, Mistry S, Michou E, Singh S, Rothwell JC, Hamdy S. Reversal of a virtual lesion in human pharyngeal motor cortex by high frequency contralesional brain stimulation. Gastroenterology 2009;137:841-849. 849 e841PMID: 19427312.


19. Verin E, Leroi AM. Poststroke dysphagia rehabilitation by repetitive transcranial magnetic stimulation: A noncontrolled pilot study. Dysphagia 2009;24:204-210. PMID: 18956227.


20. Han TR, Paik NJ, Park JW. Quantifying swallowing function after stroke: A functional dysphagia scale based on videofluoroscopic studies. Archives of physical medicine and rehabilitation 2001;82:677-682. PMID: 11346847.


21. Rosenbek JC, Robbins JA, Roecker EB, Coyle JL, Wood JL. A penetration-aspiration scale. Dysphagia 1996;11:93-98. PMID: 8721066.


22. Mullen R, Schooling T. The national outcomes measurement system for pediatric speech-language pathology. Lang Speech Hear Serv Sch 2010;41:44-60. PMID: 19833827.


23. Mullen R. Evidence for whom?: Asha's national outcomes measurement system. J Commun Disord 2004;37:413-417. PMID: 15231421.


24. Khedr EM, Abo-Elfetoh N. Therapeutic role of rtms on recovery of dysphagia in patients with lateral medullary syndrome and brainstem infarction. J Neurol Neurosurg Psychiatry 2010;81:495-499. PMID: 19828479.


25. Hamdy S, Aziz Q, Thompson DG, Rothwell JC. Physiology and pathophysiology of the swallowing area of human motor cortex. Neural Plast 2001;8:91-97. PMID: 11530891.



26. Kim BR, Kim DY, Chun MH, Yi JH, Kwon JS. Effet of repetitive transcranial magnetic stimulation on cognition and mood in stroke patients: a double-blind, sham-controlled trial. Am J Phys Med Rehabil 2010;89:362-368. PMID: 20407301.


Fig.В 1
Improvement in dysphagic parameters after repetitive transcranial magnetic stimulation (rTMS) treatment in each group. Functional Dysphagia Scale and Penetration Aspiration Scale scores improved only in the low-frequency stimulation group after rTMS (A, B). The American Speech-Language Hearing Association National Outcomes Measurements System Swallowing Scale score improved in both the sham and low-frequency stimulation groups (C). Values represent meanВұSD. FDS: Functional Dysphagia Scale, PAS: Penetration Aspiration Scale, ASHA NOMS: American Speech-Language Hearing Association National Outcomes Measurements System Swallowing Scale. *p<0.05.

-
METRICS

-
- 66 Crossref
- Scopus
- 36,073 View
- 102 Download
- Related articles in ARM