Is Palmar Cutaneous Branch of the Median Nerve More Swollen in Carpal Tunnel Syndrome?
Article information
Abstract
Objective
To investigate the characteristics of the palmar cutaneous branch of the median nerve (PCBMN) in patient with carpal tunnel syndrome (CTS) using high-resolution ultrasound.
Methods
Fourteen healthy volunteers (17 wrists) and 31 patients with CTS (41 wrists) were evaluated by high-resolution ultrasound. All patients were classified into three groups based on the electrophysiologic CTS impairment severity: mild, moderate, and severe. Using high-resolution ultrasound, the cross-sectional areas (CSAs) of the PCBMN were measured at the proximal wrist crease, bistyloid line, and distal wrist crease, and the largest CSA was defined as the maximal CSA.
Results
The maximal CSA of the PCBMN of the control, mild, moderate, and severe CTS groups were 0.27±0.08, 0.30±0.07, 0.35±0.10, and 0.47±0.13 mm2, respectively. The maximal CSA of the PCBMN was significantly larger in the severe CTS group than in the other groups.
Conclusion
The PCBMN could be concomitantly affected in patients with severe CTS.
INTRODUCTION
The palmar cutaneous branch of the median nerve (PCBMN) is the distal collateral branch of the median nerve (MN), which serves as a sensory fiber innervating the proximal palm, especially thenar eminence [1]. The MN courses down between flexor digitorum profundus and flexor digitorum superficialis muscles and take off the PCBMN around the distal forearm. The PCBMN continues down its own tunnel in the antebrachial fascia, and then passes superficial to the flexor retinaculum [2].
Traditionally, it has been known that PCBMN is spared in patients with carpal tunnel syndrome (CTS) as it does not enter the carpal tunnel. However, in a clinical setting, there are many patients with CTS who complain of symptoms such as pain, tingling, and paresthesia of the proximal palm, which suggest a lesion of the PCBMN [3,4]. In fact, several studies based on electrodiagnostic and surgical findings have been reported on concomitant entrapment neuropathy of the PCBMN in patients with CTS [5,6].
Recently, high-resolution ultrasound (HRUS) has emerged as a useful diagnostic tool for assessing the peripheral nervous system as it enables not only the morphometric characterization of individual nerves but also the quantification of nerve parameters [7-10]. Although the usefulness of HRUS for the morphometric characterization of the PCBMN has been demonstrated in several previous studies, the quantification of sonographic parameters of the PCBMN in patients with CTS has not been reported in the literature [7,11].
This study aimed to quantify cross-sectional area (CSA), a representative sonographic parameter of the PCBMN, and investigate its correlations with CTS severity.
MATERIALS AND METHODS
Participants
After obtaining written informed consent, 14 healthy volunteers (17 wrists) and 31 patients with idiopathic CTS (41 wrists) were enrolled between March 1 and September 1, 2019. This study was approved by the Institutional Review Board of Korea University Guro Hospital (No. 2020GR0307). CTS was clinically diagnosed and confirmed by an electrodiagnostic study. The exclusion criteria were as follows: trauma or surgical history of the wrist and hand, rheumatoid arthritis, degenerative joint disease involving the wrist and hand, tenosynovitis of the flexor forearm, coexistent neurologic disease (e.g., polyneuropathy, proximal median neuropathy, and cervical radiculopathy), thyroid disease, or diabetes mellitus.
Electrodiagnostic test
We performed electrodiagnosis using the Viking Select EMG/NCS machine (Nicolet Viasys, Twinsburg, OH, USA). The skin temperature of each upper limb was kept warm at ≥32°C. Nerve conduction studies were performed of the median and ulnar nerves and needle electromyography (EMG) was conducted for several muscles including the abductor pollicis brevis (APB) muscle to differentiate degree of the CTS and rule out other peripheral neuropathy or cervical radiculopathy according to standard methods. The median sensory nerve action potential (SNAP) was recorded antidromically with a surface electrode from the 3rd digit with surface stimulation along the MN conducted at two levels, 7 cm proximal to the recording electrode at the palm and 14 cm proximally at the wrist. The onset, peak latency, and baseline-tonegative peak amplitude were measured. The median compound motor action potential (CMAP) was recorded from the APB muscle with surface stimulation along the MN at two levels, 8 cm proximal to the active recording electrode and crease of the antecubital fossa. The onset latency, defined as the initial negative phase and amplitude from baseline to the negative peak were measured. We diagnosed CTS if the nerve conduction study fulfilled three of the following criteria: (1) delayed median SNAP peak latency >3.7 ms; (2) median SNAP conduction time of the proximal half segment slower than the conduction time of the other distal segment; (3) low median SNAP amplitude <20 μV; (4) a conduction block of median SNAP across wrist segment >50%; (5) delayed median CMAP distal latency >4.2 ms; and (6) low CMAP amplitude <4.5 mV. We classified CTS severity in accordance with Steven’s classification and criteria used by Lee et al. and Cho et al. [12-14].
Sonographic evaluation
Sonographic evaluation of the PCBMN was performed using the RS80A ultrasound system (Samsung Medison, Seoul, Korea) with a 3–16 MHz broad-band linear transducer. A rehabilitation medicine physician with >4 years of experience in musculoskeletal ultrasound conducted all sonographic examinations. The physician was blinded to the results of the electrodiagnostic test. We used the method devised by Jeong et al. [7], which describe the PCBMN sophisticatedly and measure the CSA of the PCBMN along its course. The subjects were seated position with elbow flexed, forearm fully supinated, and fingers slightly bent. To access the PCBMN, the rehabilitation medicine physician performed sonographic examination by manipulating the probe slowly from proximal to distal over its course. The CSAs of the PCBMN were measured at three different locations along its course: the proximal wrist crease, bistyloid line, and distal wrist crease (Fig. 1). The largest value of the CSA measured at the three locations was defined as the maximal CSA.
Statistical analyses
Data analyses were performed using IBM SPSS Statistics software (ver. 16.0.1 for Windows; IBM Corp., Armonk, NY, USA). To compare differences in the CSA of the PCBMN between control subjects and patients with CTS, an independent t-test was used. To compare differences between control subjects and patients with mild, moderate, and severe CTS, analysis of variance with post hoc analysis (Tukey’s test) was used. The p-values <0.05 were considered statistically significant.
RESULTS
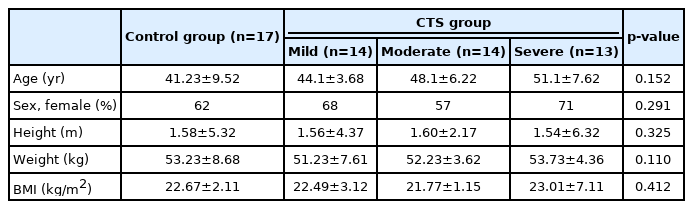
The patient demographics were similar in all the groups (Table 1). The mean maximal CSAs of the PCBMN of the control, mild, moderate, and severe CTS groups were 0.27±0.08, 0.30±0.07, 0.35±0.10, and 0.47±0.13 mm2, respectively. The maximal CSA of the PCBMN in patients with CTS was significantly greater than that of the control group (Fig. 2A). The maximal CSA of the PCBMN was significantly larger in the severe CTS group than all other groups (Fig. 2B).
DISCUSSION
In this study, we assessed the CSA of the PCBMN in patients with CTS and healthy volunteers using HRUS and investigated its relationship with CTS severity. The CSA of the PCBMN was significantly larger in the CTS group than in the control group, and the severe CTS group showed a significantly larger CSA than all the other groups.
There have been several studies on electrodiagnostic and surgical findings of concomitant entrapment neuropathy of the PCBMN in patients with CTS [5,6,15]. Imai et al. [6] compared preoperative sensory conduction studies with operative findings in patients with CTS who complained of dysesthesia of the thenar eminence and showed that the sensitivity and specificity of the electrophysiologic study were 75% and 100%, respectively, with a relatively high accuracy in demonstrating entrapment of the PCBMN. Considering that cutaneous sensory innervation of the MN and PCBMN overlaps extensively, the authors suggested that the electrophysiologic assessment of the PCBMN by measuring SNAPs is a convincing method. A study by Rathakrishnan et al. [15] showed that a slower PCBMN conduction velocity was observed in 46% of hands with CTS, which suggests concomitant PCBMN pathology. This electrodiagnostic finding was expected to help explain sensory symptoms beyond the classic sensory distribution of the MN in patients with CTS. Similarly, in a study by Uluc et al. [5], 56% patients with CTS showed abnormality of PCMBN conduction study. The results of these studies suggest that electrodiagnostic tests can help identify concomitant damage of the PCBMN in patients with CTS. In addition, the above results are consistent with our findings demonstrating that some patients with CTS also have PCBMN pathology.
With advancements in imaging technology, HRUS has emerged as an assistive modality to evaluate peripheral nerves, and several studies have been conducted to evaluate its utility in assessments of the PBCMN [7,11,16]. In a previous study, Tagliafico et al. [11] evaluated the PCBMN of 12 healthy volunteers and 22 patients complaining dysesthesia of the thenar eminence; HRUS was successfully used to identify the PCBMN in 83% and 55% patients in the normal and patient groups, respectively. Focal hypoechoic swelling of the nerve, which is consistent with our finding, was observed in patients with CTS who had a previous surgical history of carpal tunnel release. Our study differs from the above study as we obtained quantitative sonographic measures, whereas the above study described only the qualitative morphometric characteristics of the PCBMN. In a previous study by our group, we measured the quantitative parameters of the PCBMN and demonstrated a topographic position between the PCBMN and surrounding structures using HRUS [7]. We were able to identify the PCBMN in all healthy volunteers and suggested the use of HRUS to avoid iatrogenic injury during invasive procedures around the wrist and distal forearm. While the previous study was conducted in healthy volunteers alone, this study also included patients with CTS, and HRUS is still considered a useful evaluation tool in this case. To the best of our knowledge, this is the first study to assess the CSA of the PCBMN in patients with CTS according to electrodiagnostic severity using HRUS. According to our study results, the PCBMN could be concomitantly affected in patients with CTS. Therefore, the PCBMN may be a potential therapeutic target in patients with severe CTS.
Unsolved question is whether CTS and PCBMN abnormalities are independent or associated. The PCBMN transverses the wrist in long own tunnel crossing palmar carpal ligament and there is anatomical contiguity between carpal ligament and flexor retinaculum [5,15]. High pressure in the carpal tunnel may result in morphological changes of the PCBMN in the same manner as those of the ulnar nerve in Guyon’s canal combined with CTS in the author’s opinion, so concomitant PCBMN abnormalities in patient with CTS can be partially explained [17].
This study has some limitations. First, the sample size was small, limiting the generalizability of our findings. A previous study using an electrodiagnostic test examined a larger number of wrists than this study [5]. Further studies including more wrists of patients with CTS and healthy volunteers are required. Second, electrodiagnostic tests of the PCMBN were not performed in this study. Previous studies have performed electrodiagnostic tests to confirm injuries of the PCBMN [5,6]. Future studies correlating electrodiagnostic findings and sonographic features of the PCBMN could be clinically significant. Third, the diagnostic reliability may be limited because only a single clinician performed all the sonographic evaluations.
In conclusion, HRUS may be an additional diagnostic tool for assessing concomitant PCBMN abnormalities in patients with CTS. Further studies to determine the cutoff values of the CSAs for PCBMN abnormalities by sex and age are required.
Notes
No potential conflict of interest relevant to this article was reported.
Conceptualization: Jeong HM, Yoon JS. Methodology: Jeong HM, Jeong YH, Yoon JS. Formal analysis: Jeong HM. Funding acquisition: Yoon JS. Project administration: Jeong YH. Visualization: Jeong YH. Writing – original draft: Jeong HM. Writing – review and editing: Yoon SY. Approval of the final manuscript: all authors.
Acknowledgements
This research was supported by Basic Science Research Program through the National Research Foundation of Korea (NRF) funded by the Ministry of Science, ICT & Future Planning (No. NRF-2020R1A2C1009024).