Nerve Conduction Studies of Median Motor Nerve and Median Sensory Branches According to the Severity of Carpal Tunnel Syndrome
Article information
Abstract
Objective
To evaluate each digital branch of the median sensory nerve and motor nerves to abductor pollicis brevis (APB) and 2nd lumbrical (2L) according to the severity of carpal tunnel syndrome (CTS).
Methods
A prospective study was performed in 67 hands of 41 patients with CTS consisting of mild, 23; moderate, 27; and severe cases, 17. Compound muscle action potentials (CMAPs) were obtained from APB and 2L, and median sensory nerve action potentials (SNAPs) were recorded from the thumb to the 4th digit. Parameters analyzed were latency of the median CMAP, latency difference of 2L and first palmar interosseous (PI), as well as latency and baseline to peak amplitude of the median SNAPs.
Results
The onset and peak latencies of the median SNAPs revealed significant differences only in the 2nd digit, according to the severity of CTS, and abnormal rates of the latencies were significantly lower in the 2nd digit to a mild degree. The amplitude of SNAP and sensory nerve conduction velocities were more preserved in the 2nd digit in mild CTS and more affected in the 4th digit in severe CTS. CMAPs were not evoked with APB recording in 4 patients with severe CTS, but obtained in all patients with 2L recording. 2L-PI showed statistical significance according to the severity of CTS.
Conclusion
The branch to the 4th digit was mostly involved and the branch to the 2nd digit and 2L were less affected in the progress of CTS. The second digit recorded SNAPs and 2L recorded CMAPs would be valuable in the evaluation of severe CTS.
INTRODUCTION
Carpal tunnel syndrome (CTS) is a neuropathy caused by entrapment of the median nerve at the wrist, which leads to pain, hypesthesia at the dermatome area and weakness of the abductor pollicis brevis (APB) muscle [1]. It is the most common peripheral neuropathy and Atroshi et al. [2] reported that it occurs in 5.8% of women and 0.6% of men.
Diagnosis of CTS is made with clinical symptoms, physical examination and electrodiagnostic study, of which, electrodiagnostic study is known to be the most accurate and useful method of diagnosis [3,4]. However, a variety of study methods exist to increase the sensitivity, and every physician has his own preferred method. Measuring the differences in peak, onset latency and baseline to peak amplitude between stimulation at the wrist, where a 14 cm proximal to the recording electrode and at the palm, 7 cm proximal to the electrode, and comparing latencies with ulnar and radial sensory nerve are the general methods recommended by the American Association of Neuromuscular Electrodiagnostic Medicine [5,6]. In most cases, the 2nd or 3rd finger is used for recording electrodes in a median nerve sensory conduction study [3,5]. However, the debate in which the finger is most sensitive for the diagnosis of CTS is still ongoing [7-9].
Sheean et al. [6] reported that the onset latency of median motor conduction studies recorded on APB is less sensitive than other electrodiagnostic parameters in CTS diagnosis, and that it would be useful to compare the onset latencies of compound muscle action potentials (CMAP) of the median innervated lumbrical muscles and ulnar innervated interosseous muscles in the palm. Boonyapisit et al. [10] also reported that for a moderate degree of CTS, it is helpful to use a lumbrical recording for localization of the lesion and differential diagnosis from other conditions, such as polyneuropathy, when action potential is not evoked due to atrophy of APB.
In this study, we evaluated the median sensory nerve action potentials (SNAP) from each digit and CMAP onset latency differences between the 2nd lumbrical muscle and interosseous muscle for each CTS severity to observe the involvement of median sensory and motor nerve branches, according to disease progression.
MATERIALS AND METHODS
Materials
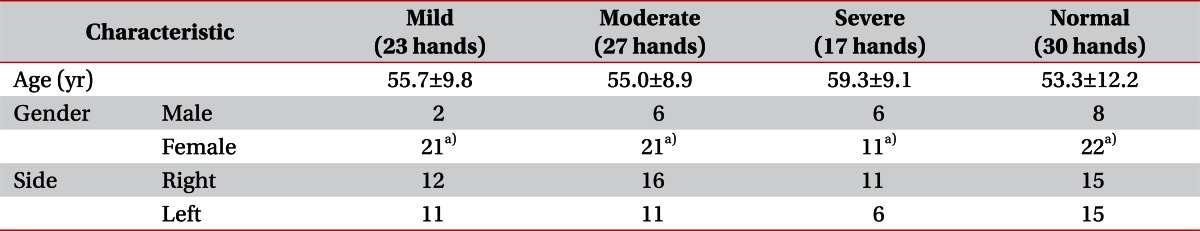
A prospective study was performed on 67 hands of 41 patients who visited Korea University Hospital for tingling sensation, pain and hypesthesia at the median nerve dermatome and were diagnosed with CTS in an electrodiagnostic study. The average age was 56.2±11.2 years with 9 males (14 hands) and 32 females (53 hands). Patients with peripheral polyneuropathy, ulnar neuropathy, and cervical radiculopathy were excluded. Patients who had received surgical management for diagnosed CTS were also excluded. To obtain the criteria for amplitude ratios of the sensory nerve conduction study, 21 control subjects without any clinical symptoms or history of surgical management were also evaluated.
Methods
Counterpoint Mk2 (Dantec, Skovlunde, Denmark) was used for the electrodiagnostic study. An antidromic method was used for the sensory nerve conduction study. For the median sensory conduction study, an active electrode was placed on the proximal phalanx at each of the 1st to 4th digits, and the reference electrodes were placed 4 cm distal to each active electrode. Stimulation was applied at 10 cm proximal [11] to the active electrode for the 1st digit, and 14 cm proximal to the 2nd, 3rd, and 4th digits. For the ulnar sensory conduction study, active, and reference electrodes were placed at the 4h and 5th digits, and stimulation was applied at the wrist 14 cm proximal to the active electrode. For every SNAP, the onset latency, peak latency, baseline to peak amplitude, and conduction velocity was measured. The median nerve peak latencies of over 3.7 ms at 2nd, 3rd, and 4th digits, were considered as prolonged latency.
For the motor conduction study, an active electrode was placed at the middle portion of the APB muscle and stimulation was applied at the wrist, 8 cm proximal to the active electrode and at the elbow. Onset latency, amplitude and conduction velocity were obtained. Preston's method [12] was used for the lumbrical and 2nd interosseous muscle recordings. An active electrode was placed at the lateral portion of the 3rd metacarpal bone, as well as a reference electrode at the 2nd metacarpal joint. Stimulation was applied at the wrist 10 cm proximal to the active electrode for the median and ulnar nerve.
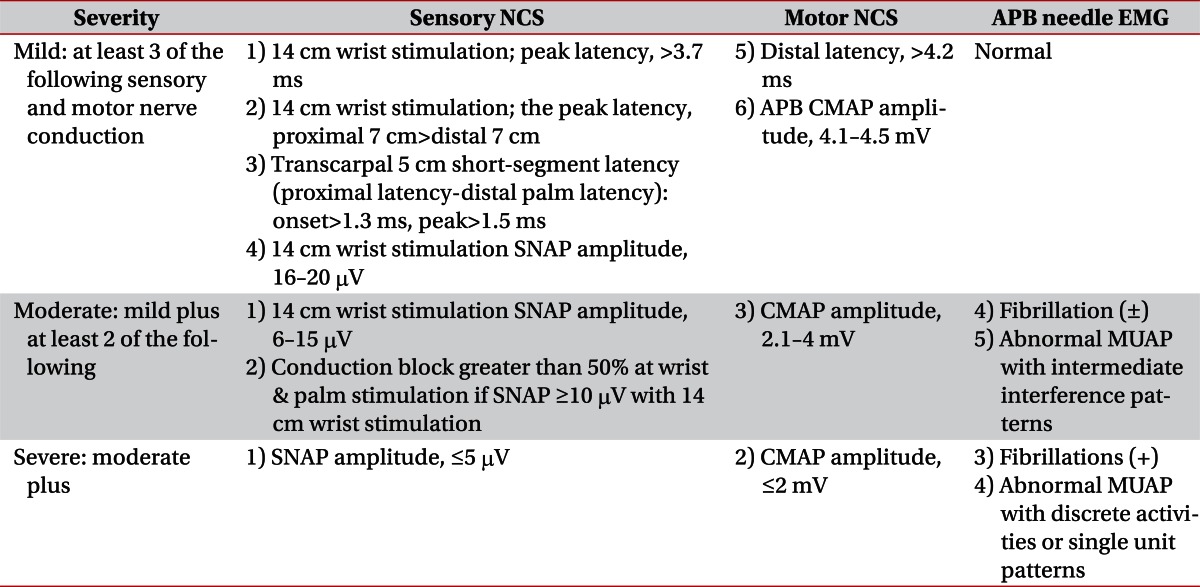
Criteria using the 3rd digit, revised by the author, were used for CTS severity classification. For the 2nd digit, the same criteria were applied for the onset latency, peak latency, and baseline to the peak amplitude and conduction velocities. For the 4th digit, amplitude was excluded from the criteria (Table 1) [13]. For the 1st digit, considering the difference in distance between the electrodes from the other digits, latency difference according to the severity was evaluated within the 1st digit, and the sensory conduction velocity was compared with the 2nd, 3rd, and 4th digits. For the median motor conduction study, an onset latency over 4.2 ms and lumbrical-interosseous latency difference over 0.4 ms were considered as abnormal [6].
Parameters analyzed in the median sensory conduction study were onset latency, peak latency, baseline to peak amplitude and conduction velocity from each digit. In analyzing SNAP amplitude, due to a considerable number of bilateral CTS, amplitude ratios of the median SNAP from the 2nd and 3rd digits to ulnar SNAP from the 5th digit were obtained. Due to dual innervation at the 4th digit, the median SNAP amplitude was small even in normal subjects. Hence, amplitude ratios to the ulnar SNAP at the 4th digit were obtained. For amplitude ratio analysis, criteria obtained from the receiver operating characteristic (ROC) curve was compared with the patient group. Frequency of abnormal values for amplitude ratio was evaluated. Criteria for the 2nd and 3rd digit were 0.8 and for the 4th digit was 0.83, respectively. Due to the short distance between the electrode and the stimulation site, amplitude comparison was made only between the 2nd, 3rd, and 4th digits, and the 1st digit was excluded. For median CMAP parameters, onset latency from APB, 2nd lumbrical muscle and lumbrical-interosseous latency differences were analyzed. For the subjects with unevoked potentials, predictive values for onset latency and conduction velocity, corresponding to an amplitude value of '0' using regression analysis was used as a substitute value [14].
SPSS ver. 12.0 (SPSS Inc., Chicago, IL, USA) was used for statistical analysis. Age difference and comparison with the control group according to the CTS severity was analyzed using one-way analysis of variance (ANOVA) test and Tukey test for the post hoc study. For other discrete variables, the chi-square test or Fisher exact test was used. Changes in the median SNAPs of the four digits, according to the CTS severity were analyzed using repeated measures of ANOVA. Criteria were obtained with an amplitude ratio of the control group, using the ROC curve. Based on the criteria, the frequencies of which abnormal values appear for each digit were analyzed in the patient group. A p-value less than 0.05 was considered to be statistically significant.
RESULTS
Among the 67 hands of the 41 patients diagnosed with CTS, 23 were of mild degree, 27 moderate, and 17 were severe degree. The average age of the entire CTS patient group was 56.2±11 years, and 55.7±9.8 years in the mild degree group, 55.0±8.9 years in the moderate degree group, 59.3±9.1 years in the severe degree group, and 53.3±12.2 years in the control group. The female ratio was higher in both the patient and the control groups. No statistical significance was observed with regard to which hand was involved. There were no differences with respect to age according to the CTS severity (Table 2).
Median SNAP latency comparison
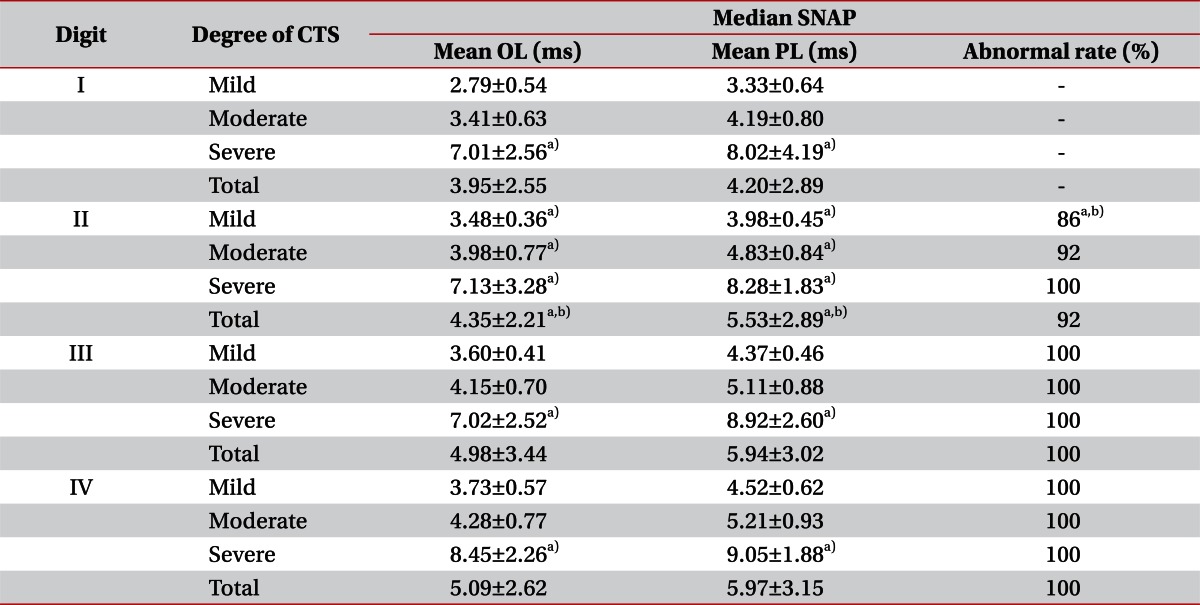
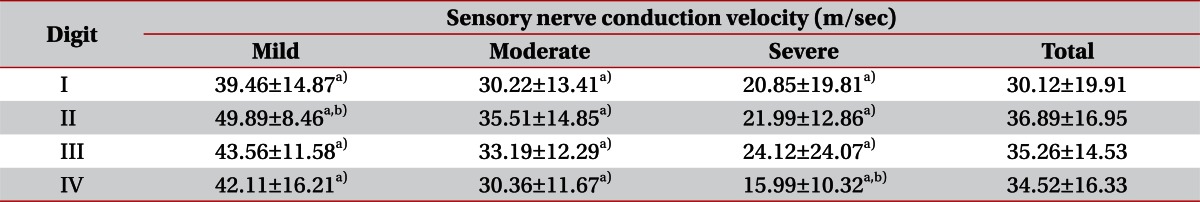
The average onset and peak latency of the patient group for each digit were: 3.95±2.55 and 4.20±2.89 ms for the 1st digit, 4.35±2.21 and 5.53±2.89 ms for the 2nd digit, 4.98±3.44 and 5.94±3.02 ms for the 3rd digit, and 5.09±2.62 and 5.97±3.15 ms for the 4th digit, respectively. Of the three digits with the same distance between an electrode and the stimulation site, the 2nd, 3rd, and 4th digits, the latency of the second digit was shorter with statistical significance (p<0.05), where the 4th digit showed the most prolonged latency without statistical significance. Onset and peak latency differences according to the CTS severity for each digit showed statistical differences in mild, moderate, and severe degree groups for the 2nd digit (p<0.05). For the 1st, 3rd, and 4th digits, only the moderate degree group showed a significant latency prolongation (p<0.01). In the 2nd digit, frequency of peak latency prolongation was significantly lower in the mild group compared to the moderate and the severe groups (p<0.05). In the 3rd and 4th digits, latency was prolonged in all CTS degree groups and the frequency of latency prolongation was significantly lower in the 2nd digit compared to the 3rd and 4th digits (p<0.01) (Table 3). Among the patients diagnosed with severe CTS, SNAP was not evoked on the 1st, 3rd, and 4th digits on 8 hands. On 6 of the 8 hands, SNAP was not evoked on the 1st, 2nd, 3rd, and 4th digit, yet 2 hands evoked SNAP on the 2nd digit.
Median SNAP amplitude comparison
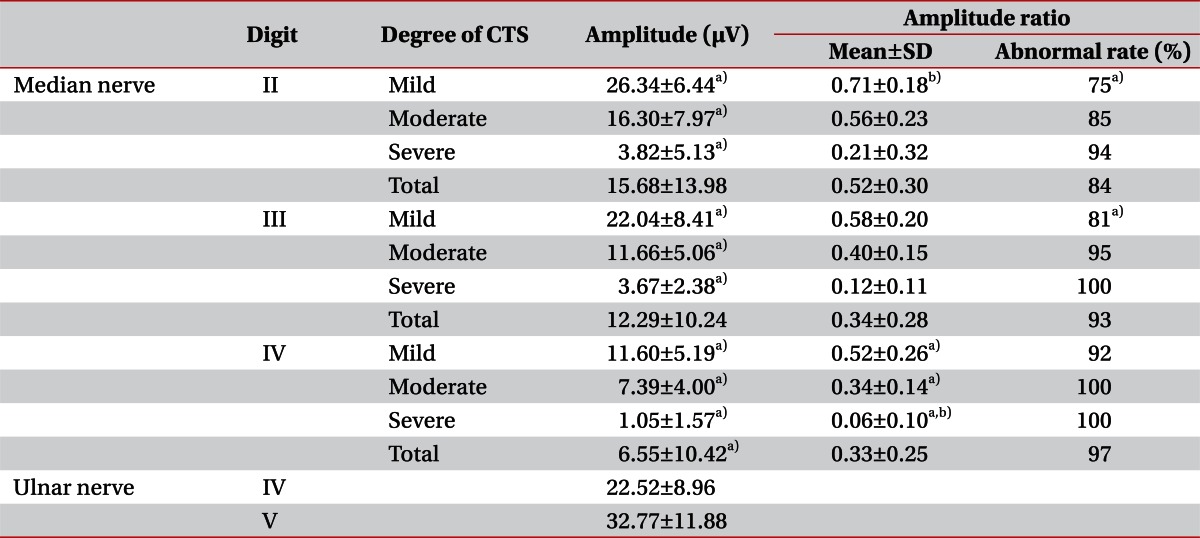
All the digits showed a decrease in the median SNAP amplitude according to the disease severity (p<0.05). However, in the comparison of amplitude ratios according to the disease severity, only the 4th digit showed significant amplitude ratio differences between the mild, moderate, and severe groups (p<0.05). The 2nd and 3rd digits showed decreases in amplitude ratios with the disease severity, without statistical significance. In comparing the median nerve amplitude ratios between the digits for each severity, the 2nd digit ratio was significantly larger in the mild group (p<0.01) and the 4th digit ratio was significantly smaller in the severe group (p<0.01). Criteria obtained from the amplitude ratios of the control group were 0.89 and 0.88 for the 2nd and 3rd digits, and 0.86 for the 4th digit. The frequency of abnormal amplitude ratios in the patient group were 75% for the 1st, 81% for the 2nd and 3rd digits in the mild group, which was significantly lower than for the moderate and the severe groups (p<0.05). In a comparison between digits, frequency was lowest with the 2nd digit and highest in the 4th digit, without any statistical significance (Table 4).
Comparison of median nerve conduction velocity
By comparison, a significant decrease in conduction velocity according to the disease severity was observed in all the digits (p<0.05). Comparing the digits for each severity, the conduction velocity was preserved more with the 2nd digit in the mild degree group (p<0.05) and more impaired with the 4th digit in the severe group (p<0.05). The conduction velocity of the 1st digit was decreased compared to the other groups in the patient group, without statistical significance (Table 5).
Comparison of median CMAP
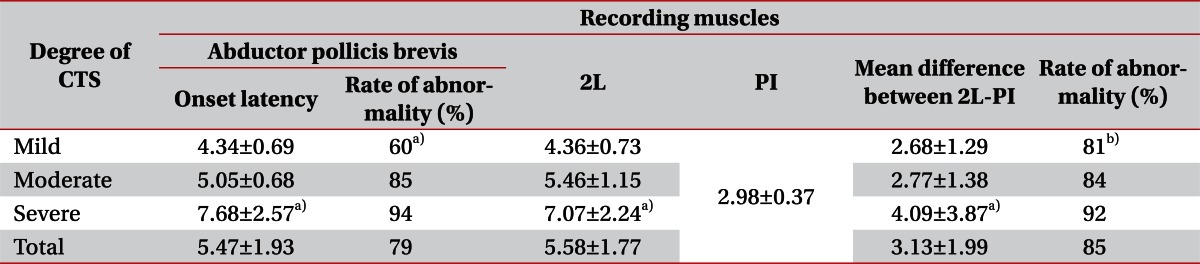
The onset latency of APB recording and lumbrical muscle recording was significantly prolonged in the severe group compared to the mild and moderate degree groups. Frequencies of onset latency prolongation in the APB recording were 60%, 85%, and 94% in the mild, moderate, and severe groups, respectively. Frequency in the mild group was significantly lower. Lumbrical-interosseous latency difference was 2.68±1.29 ms in the mild group and 2.55±1.38 ms in the moderate group, showing no significant difference. However, in the severe group, the latency difference was 4.09±3.87 ms with statistical significance compared to the other groups (p<0.05). The frequency of latency differences longer than 0.4 ms were 81%, 84%, and 92% in the mild, moderate, and severe group, respectively. The frequencies showed a tendency to rise according to the severity, buy without any statistical significance. In the mild group, frequency of lumbrical-interosseous latency differences longer than 0.4 ms were significantly higher than the frequency of APB recording latency, longer than 4.2 ms (p<0.05). In the moderate and severe groups, no difference was observed between the two frequencies. In the 4 digits, whose CMAPs were not evoked with the APB recording, CMAPs were all observed with a lumbrical recording (Table 6).
DISCUSSION
An electrodiagnostic study is used as an objective and accurate tool in diagnosing CTS; the sensory conduction study is known to be more sensitive than the motor conduction study in the early stage of the disease [7,15]. However, various studies reported controversial results, regarding which digit should be tested in order to increase the sensitivity and accuracy of the test. Aydin et al. [7] performed studies in comparing the median nerve conduction velocities of the 1st, 2nd, 3rd, and 4th digits on 818 hands of 525 CTS patients. This study reports that the 1st digit showed the highest frequency of decreased conduction velocity and that the 2nd digit conduction velocity was relatively preserved. A study by Tsaiweichao-Shozawa et al. [15] states that the sensory branch of the median nerve to the 2nd digit and motor branch to the lumbrical muscle is relatively preserved in the late course of the disease. In the study performed with 55 CTS patients, Macdonell et al. [16] reported that the decrease in median conduction velocity was most prominent with the 1st digit and was less frequent with the 2nd digit. However, Padua et al. [17] reported that there are significant decreases in conduction velocity at the 1st and 3rd digits without a significant difference between the two digits. Lauritzen et al. [18] also reported that there is no difference in the sensitivity of the test between the 1st and 3rd digit in a mild degree CTS. In a study by Terzis et al. [8] with 72 patients with mild degree CTS whose onset latency was shorter than 4.2 ms with APB recording, reported that the sensitivity of conduction studies on the 1st, 2nd, 3rd, and 4th digits were be 61%, 22%, 50%, and 88%, respectively-the 4th digit being the most useful in CTS diagnosis. Cioni et al. [19] compared the frequency of lowered conduction velocities between the digits, in a group of patients with clinical symptoms of CTS, where the frequencies were 64%, 80%, and 92% in the 1st, 2nd, and 3rd digits, respectively. Conduction velocity was lowered in all of the patients in the 4th digit.
In this study, comparisons of each digit according to the disease severity showed significant differences in the 2nd digit compared to the 1st, 3rd, and 4th digits for the mild, moderate, and severe groups. It seems that such a difference is due to the well preserved sensory branch to the 2nd digit in the mild degree group, which showed a difference even with the moderate group. Frequency of latency prolongation was significantly lower with the 2nd digit and SNAP amplitude ratio was also significantly higher compared to the 3rd and 4th digits. Frequency of abnormal values was also lower compared to the control group, though without statistical significance. The fact that among the 8 hands whose SNAPs were not evoked with the 1st, 3rd, and 4th digits, the fact that 2 showed preserved SNAPs with the 2nd digit also supports these results. By contrast, the amplitude ratio was significantly low with the 4th digit, and the frequency of abnormal value was higher compared to the other digits in all the severities without statistical significance. An evaluation on the conduction velocity showed similar results. In the mild degree group, conduction velocity was relatively preserved with the 2nd digit, and in the severe group, it was significantly decreased with the 4th digit. In comparison of the conduction velocities despite the severity degree, it was decreased with the 1st digit, compared to the other digits.
Considering all the results from this study, it can be said that in the early stage of CTS, the 2nd digit is preserved. Differences in the degree of impairment between the digits according to the disease progression can be explained by fascicular arrangement of the median nerve under carpal tunnel [16,20]. In CTS, the median nerve injury occurs most severely at the distal end of carpal tunnel, just proximal to which the motor and sensory branches divide. In carpal tunnel, the median sensory branches to the 1st and 3rd digits lie in the antero-lateral side, branches to the 4th digit lie in the antero-medial side, and to the 2nd digit lie in the postero-medial side. Due to such a fascicular arrangement, sensory branches to the 2nd digit, located in the posterior side, are less vulnerable to injury compared to other digits. Moreover, conduction study values are more preserved in the early stage, when compression or ischemia occurs in the carpal tunnel [7].
Despite the fact that the 4th digit is severely affected by the disease progression, in clinical practice, physicians prefer to use the 2nd and 3rd digits over the 4th digit in median sensory conduction studies, due to small SNAPs caused by dual innervation of the median and ulnar nerve. However, considering the fact that the sensitivity of the 2nd digit in CTS diagnosis is not high [7,15,16], and a study by Padua et al. [17] reported that the distal-proximal distance ratios of the elbow and hand according to the course of the median nerves is most ideal in the 2nd and 3rd digits, it is most appropriate to use the 3rd digit for the active recording electrode in CTS diagnosis. In this study, diagnostic criteria for the peak latency was set to 3.7 ms for the 2nd, 3rd, and 4th digits considering the same distance applied between the electrode and the stimulator. Considering different degrees of disease involvement in each digit, according to the disease progression, applying the same criteria can be a limitation of this study.
We also performed evaluations on the median motor conduction study. Among the median motor branches, the recurrent branch to the APB muscle is in a position to be more vulnerable to carpal tunnel compression than branches to the 1st and 2nd lumbrical muscles. Sunderland [21] discovered that branch to the lumbrical muscle is positioned postero-medially to the APB branch at 37 mm distal to the radial styloid process of the wrist, and at the 20 mm distal point, it is positioned relatively anterior compared to the distal part, yet still posterior to the APB branch. They also reported that the sensory branch to the 2nd digit is positioned relatively posterior to the lumbrical muscle motor branches. Tsaiweichao-Shozawa et al. [15] reported that due to such arrangements of nerve fascicles, branches to the 2nd digit and lumbrical muscle are relatively more preserved in carpal tunnel compression, and that a correlation exists between the conduction study values of the two branches. Therefore, various studies exist on the clinical usefulness of lumbrical muscle recording for CTS diagnosis. Sheean et al. [6] claimed that though using lumbrical muscle may not increase the sensitivity of electrodiagnostic study, measuring lumbrical-interosseous muscle latency differences can be useful. Boonyapisit et al. [10] reported that in moderate degree CTS, a lumbrical muscle recording conduction study is useful in the dfferential and accurate diagnoses.
In this study, latency prolongation with APB muscle recording was significantly lower in frequency than lumbrical-interosseous latency differences in the mild degree group, which is similar to a previous study by Sheean et al. [6] The reason for such result is that in the early process of CTS, demyelination is the main pathology of the disease, so when the damage is minimal, latency may appear within normal range. However, compared to the latency of the ulnar nerve, which does not pass through the carpal tunnel, latencies of the median nerve is relatively prolonged and the latency difference can be significant, which makes lumbrical-interosseous latency differences more sensitive than the latency recorded from the APB muscle. As the disease progresses, the pathophysiology of the disease changes and due to its anatomically favorable position, the branch to the lumbrical muscle is less affected by the conduction block or axonal injury, and is relatively preserved in the conduction study. In this study, from the 4 digits with unevoked APB recorded CMAPs, and from the 8 digits with unevoked SNAPs, lumbrical muscle recording CMAPs were all obtained. These results show that the lumbrical-interosseous latency difference is a tool for increasing sensitivity in CTS diagnosis, and consistent with previous studies, as the disease progress, lumbrical muscle is relatively preserved in severe degree CTS.
In conclusion, this study shows that the 4th digit SNAP is the most severely affected in CTS, but the 2nd digit SNAP is relatively spared, and that lumbrical recorded median CMAP is spared in the severe degree CTS group. Considering its sensitivity and specificity, the 3rd digit is commonly used in clinical practice for electrodiagnostic study in CTS. However, in severe patients, using the SNAP recorded on the 2nd digit or the CMAP recorded on the lumbrical muscle, which are relatively spared even in the late stage of the disease, may help in diagnosing CTS.
Notes
No potential conflict of interest relevant to this article was reported