Alien Hand Syndrome in Stroke - Case Report & Neurophysiologic Study -
Article information
Abstract
Alien Hand Syndrome is defined as unwilled, uncontrollable, but seemingly purposeful movements of an upper limb. Two major criteria for the diagnosis are complaint of a foreign limb and complex, autonomous, involuntary motor activity that is not part of an identifiable movement disorder. After a cerebrovascular accident in the corpus callosum, the parietal, or frontal regions, various abnormal involuntary motor behaviors may follow. Although different subtypes of Alien Hand Syndrome have been distinguished, this classification clearly does not cover the wide clinical variety of abnormal motor behaviors of the upper extremity. And there are few known studies about the neurophysiology of this syndrome using transcranial magnetic stimulation (TMS). We recently experienced 2 rare cases of Alien Hand Syndrome which occurred after anterior cerebral artery (ACA) infarction. A 72 year-old male with right hemiplegia following a left ACA infarct had difficulty with voluntarily releasing an object from his grasp. A 47 year-old female with left hemiplegia following a right ACA infarct had a problem termed 'intermanual conflict' in which the two hands appear to be directed at opposing purposes. Both of them had neurophysiologic studies done, and showed reduced amplitude by single pulse MEP and a lack of intracortical inhibition (ICI) by paired pulse TMS. No abnormalities were found in SSEP.
INTRODUCTION
Alien Hand Syndrome (AHS) is a syndrome where patients with brain injury report involuntary movement, especially of their upper limbs. In 1908, Goldstein first described this syndrome as "a type of apraxia with the feeling of estrangement between the patient and his hand".1 It is a rare condition that can be caused by stroke, midline tumors, or callosotomy. Traditional studies classify AHS into two different categories, both from a clinical and anatomical perspective: a frontal AHS and a callosal AHS.2 In this type of AHS, motor circuits are abnormally activated. On the contrary, a sensory disorder after posterior cerebral artery infarction is distinctively considered as sensory AHS.3 Although several studies have reported on patients with AHS, there are very few studies about the neurophysiology of this syndrome.
We recently experienced two rare cases of AHS that occurred mality of these two cases using somatosensory evoked potential (SEP) and transcranial magnetic stimulation (TMS) with a review of the literature. after anterior cerebral artery (ACA) infarction. In this report, we describe the neurophysiologic abnormality of these two cases using somatosensory evoked potential (SEP) and transcranial magnetic stimulation (TMS) with a review of the literature.
CASE REPORTS
Case 1
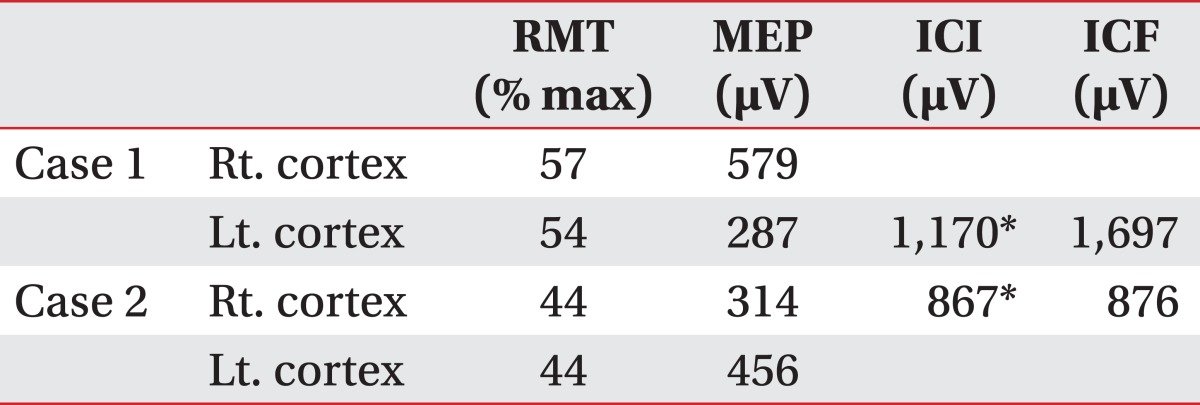
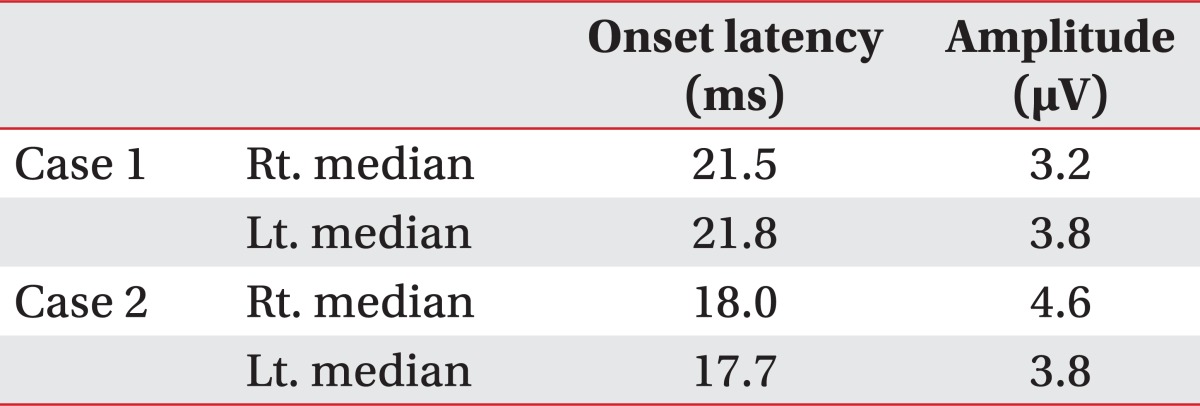
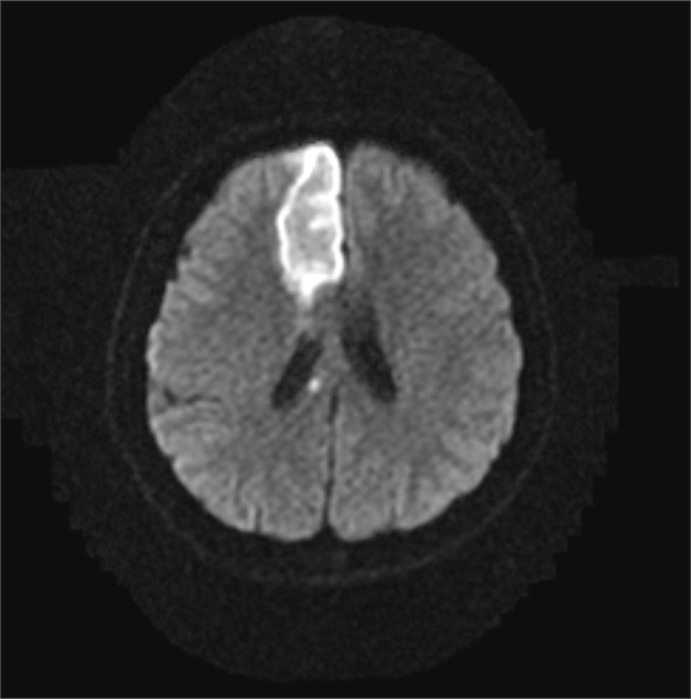
A 72 year-old man developed right side weakness after a left ACA infarction and was transferred to the department of rehabilitation medicine 7 weeks after the attack. He had no remarkable medical history but was diagnosed with diabetes at our hospital. He had a family history of ischemic stroke (affecting his younger brother). On transfer to our department, he was alert but had cognitive impairment with a K-MMSE (Korean version of the Mini-Mental Status Examination) score of 13. Manual muscle test (MMT) grades of his right upper extremity-were all 3/5, lower extremity test grades were 2/5 except for ankle plantar flexor grades of 1/5, and dorsiflexor grades were 0/5. He had no spasticity in his elbow and knee joints, and had a modified Ashworth scale (MAS) score of zero. His sensory function was intact and deep tendon reflex was normoactive. He complained that his hand moved abnormally against his will. He could grasp and release with his bare hands on the doctor's order, but when grasping some objects with his palm, he was unable to release them. Brain magnetic resonance imaging (MRI) revealed acute cerebral infarction at the left ACA region (Fig. 1). For TMS, we used a Magstim 200 monopulse® (Magstim co., Whiteland, UK) connected to a figure-eight coil (7 cm outer diameter for each loop) with a maximum magnetic strength of 2.0 tesla. The coil was initially centered on the vertex, perpendicular to the scalp, with the handle pointing posteriorly at a 45 degree angle from the median sagittal line and then moved in 1 cm steps in anterior-posterior and medial-lateral directions. Motor evoked potentials (MEP) were recorded from the first dorsal interosseous (FDI) muscle with a surface gel electrode. The active electrode was placed over the muscle belly and the reference electrode over the distal interphalangeal joint of the index finger. The signals were displayed by Keypoint® EMG (Dantec, Skovlunde, Denmark) and stored in a laboratory computer. Using a paired pulse magnetic stimulation technique, we studied intracortical inhibition (ICI) and intracortical facilitation (ICF). In this case, when we stimulated the left motor cortex area, the resting motor threshold (rMT) was 54% of the maximum magnetic strength and the MEP amplitude was 287 µV. At the right motor cortex area, the rMT was 57% and the MEP amplitude was 579 µV. In the ICF study, when we stimulated the left motor cortex area, the MEP amplitude was increased to 1,697 µV. In the ICI study, the amplitude was not suppressed, but rather increased to 1,170 µV (Table 1). The SEP studiesusing median nerve stimulation showed normal values with no statistically significant difference between sides (Table 2).

Axial magnetic resonance image showing a high signal and indicating an acute infarction in the left frontal periventricular white matter on a diffusion weighted image (Case 1).
Case 2
A 47 year-old woman developed left side weakness after a right ACA infarction and was transferred to the department of rehabilitation medicine 3 weeks post onset. She had no remarkable medical history but was diagnosed with diabetes at our hospital. She had no family history of stroke. On transfer to our department, her mental status was alert but she had mild attention deficit. Her speech was normal and a K-MMSE score was 25. MMT grades of her left upper and lower extremities were all 3/5, except the wrist flexor and wrist extensor scores were both 2/5. She could perform fine motor activity with her left hand. She had no spasticity in her elbow and knee joints, with a MAS score of zero. Her deep tendon reflex was normoactive. Her superficial sensory responses were intact, but proprioception was impaired. She complained that her left hand was out of her control; her left hand "took away" objects out of her right hand. Her hands appeared to be directed at opposing purposes, showing 'intermanual conflict'. A brain MRI revealed acute cerebral infarction at the right ACA region, mainly at the right frontal lobe and corpus callosum (Fig. 2). In this case, when we stimulated the right motor cortex area, the rMT was 44% and the MEP amplitude was 314 µV. At the left motor cortex area, the rMT was 44% and the MEP amplitude was 456 µV. In the ICF study, when we stimulated the right motor cortex area, the MEP amplitude was increased to 876 µV. In the ICI study, theamplitude was not suppressed, but rather increased to 867 µV (Table 1). The SEP studies using median nerve stimulation were normal with no significant differences between sides (Table 2).
DISCUSSION
A precise definition of the AHS does not yet exist. Hallmarks of the AHS include: first, a feeling of foreignness of the limb; second, failure to recognize ownership of the limb when visual clues are removed; third, autonomous motor activities that are perceived as involuntary and are different from other identifiable movement disorders and fourth, personification of the affected body part.2 Since Goldstein's initial description, there were several case reports of AHS. Most of them came after a corpus callosum or frontal lobe lesion, either alone or in combination. And there were some cases reporting AHS after damage in an occipital lobe or in a parietal lobe. The callosal AHS involves the non-dominant hand and is characterized primarily by intermanual conflict. The callosal-frontal AHS shows more grasping behavior and complaints of compulsive manipulation. The posterior form generally involves the non-dominant hand, which tends to levitate into the air displaying ataxia, with relevant sensory impairment.4
The course of AHS and its prognosis has not been systematically reported. The prognosis has varied from a decrease in AHS symptoms within 1 week to persistence of the symptoms after 12 months. In a literature review, decreases in symptomsoccurred in 68% of patients, whereas symptoms persisted in 32%.1
The ACA supplies the rostral sensorimotor cortex and the anterior two-thirds of the corpus callosum. ACA occlusion may disrupt interhemispheric connections.Proximal occlusion may not only damage the motor cortex, resulting in weakness of the contralateral limbs, but may also produce other abnormal motor phenomena. These include compulsive movements of a reflex nature, described as a grasp reflex. Abnormal motor behavior of a more complex and semi-purposive nature may also occur in the contralateral upper limb and has been described as the alien hand sign. In extreme cases, where one hand acts at cross-purposes to the other, the term 'intermanual conflict' has been used to describe this behavior.5
Case reports of AHS in three patients with tumors of the corpus callosum introduced the concept that damage in the callosal connection can be the cause of this syndrome.5 The corpus callosum is a bundle of neural fibers, consisting of white matter structures in the brain and connects the left and right cerebral hemispheres. In two other cases with a left medial frontal cortex infarction, the right hand displayed AHS. The authors believe that these abnormal forms of motor behavior were the result of damage to the medial frontal cortex, which includes the supplementary motor area.5
There are several studies about the brain lesion that can cause AHS, but there are very few known studies about the neurophysiology of this syndrome. In this case study, we observed AHS after an acute ACA infarction, which mainly caused intermanual conflict and a grasp ing reflex. To study neurophysiologic change in this syndrome, we used the TMS technique. Recently, using paired TMS, ICI is being studied to examine the bal ance between intracortical excitatory and inhibitory mecha nisms. Among healthy volunteers, paired TMS with short interstimulus intervals (ISIs) (1-4 ms) suppress MEP amplitude, and with long ISIs (8-15 ms) MEP amplitude increase.6 When we examined a patient with Parkinson's disease using this method, the ICI was disinhibited compared to healthy persons. A study about specific dystonia, namely writer's cramp, and musician's dystonia, showed that inhibition in the motor cortex is abnormal, and these abnormalities were seen in both hemispheres.7 In a study with patients in a subacute stage after stroke, ICI was significantly disinhibited and the ICF showed reduced facilitation at the motor cortex of the affected side.8 These findings were contrary to our findings, showing disinhibition of the ICI and also increased facilitation in the ICF of the motor cortex of the affected side.
For healthy volunteers, fine motor activity needs inhibition of nearby muscles when those muscles are not involved in doing activity.9 And, according to this theory, our findings of disinhibition in ICI can be the cause of the abnormal hand movement, such as dystonia or AHS. But, in focal hand dystonia, it is combined with sensorimotor impairment10 and in our cases there was no SEP abnormality. We can postulate that dystonia and AHS may have different neurophysiologic mechanisms. In classic callosal AHS and frontal AHS, we studied SEP and TMS and found some abnormalities. With more case studies, we expect that neurophysiologic mechanisms of AHS can be found in the near future.
ACKNOWLEDGEMENTS
This work was supported by Inha University Research Grant.