Association Between Latency of Dermatomal Sensory-Evoked Potentials and Quantitative Radiologic Findings of Narrowing in Lumbar Spinal Stenosis
Article information
Abstract
Objective
To identify the difference of quantitative radiologic stenosis between a normal latency group and an abnormal latency group, and to investigate the association of dermatomal somatosensory-evoked potential (DSEP) with magnetic resonance imaging (MRI) findings of narrowing in patients with lumbar spinal stenosis (LSS).
Methods
We retrospectively reviewed the clinical records and P40 latencies of L5 DSEP of 40 patients with unilateral symptoms of LSS at the L4–5 disc level. Quantitative assessments of stenosis in lumbar spine MRI were performed with measurements of the anteroposterior diameter (APD), cross-sectional area (CSA) of the dural sac, ligamentous interfacet distance (LID), CSA of the neural foramen (CSA-NF), and subarticular zone width. Analyses were conducted through comparisons of radiologic severity between the normal and abnormal latency groups and correlation between radiologic severity of stenosis and latency of DSEP in absolute (APD <10 mm) and relative (APD <13 mm) stenosis.
Results
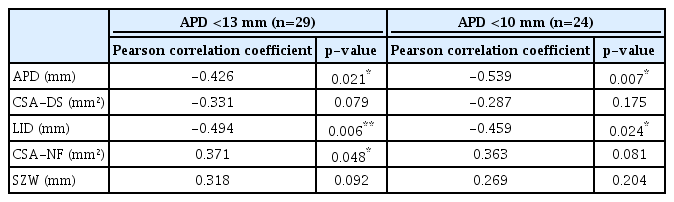
The radiologic severities of lumbar stenosis were not significantly different between the normal and abnormal latency groups. In absolute and relative stenosis, latency showed a significant negative correlation with APD (r=-0.539, r=-0.426) and LID (r=-0.459, r=-0.494). In patients with relative stenosis, a weak significant positive correlation was found between latency and CSA-NF (r=0.371, p=0.048). LID was the only significant factor for latency (β=-0.930, p=0.011).
Conclusion
The normal and abnormal DSEP groups showed no significant differences inradiologic severity. The latency of DSEP had a negative correlation with the severity of central stenosis, and LID was an influencing factor.
INTRODUCTION
Lumbar spinal stenosis (LSS) is one of the most common degenerative diseases of the spine, especially in old age. LSS has been defined as “buttock or lower-extremity pain, which may occur with or without low back pain, associated with diminished space available for the neural and vascular elements in the lumbar spine” [1]. Disc degeneration, facet degeneration and hypertrophy, degenerative spondylolisthesis, and ligamentum flavum hypertrophy and calcification can cause LSS, alone or in combination [2]. Compression caused by both central stenosis and lateral stenosis is believed to contribute to neurogenic claudication associated with LSS [3]. As stenosis is found in many levels and L4–5 is the most frequently involved level [4,5], the L5 root is most likely to be involved. Despite its high prevalence, there is no generally accepted gold standard method for the diagnosis of LSS. A proper diagnostic method is essential to prevent false-positive results, which can lead to unnecessary treatments [6].
When a clinical impression of LSS is made, the diagnosis is confirmed on the basis of the finding of “narrowing” or “stenosis” of the spine on imaging modalities such as computed tomography or magnetic resonance imaging (MRI). The North American Spine Society states in their guidelines that imaging is the key non-invasive test for LSS, and MRI is the most commonly used imaging method [1]. Nevertheless, its role remains controversial. Wassenaar et al. [7] summarized the diagnostic accuracy of MRI for lumbar disc herniation and spinal stenosis, and suggested that the evidence for the diagnostic accuracy of MRI is inconclusive. Another review article on the radiologic signs of LSS concluded that there is a need for consensus on well-defined, unambiguous radiologic criteria to define LSS [8]. A Delphi survey of radiologic criteria for the diagnosis of LSS concluded that “there are no broadly accepted quantitative criteria and only partially accepted qualitative criteria for the diagnosis of LSS” [9]. Nevertheless, constant efforts have been made for evaluating LSS based on quantitative radiologic findings. In a report dealing with radiologic criteria and parameters for LSS, the anteroposterior diameter (APD) of the spinal canal, cross-sectional area of the dural sac (CSA-DS), ligamentous interfacet distance (LID), and others were included as parameters for central stenosis, and lateral recess height, depth of the lateral recess, and lateral recess angle were included as parameters for lateral stenosis [8,9]. Absolute stenosis is defined as APD of the spinal canal <10 mm, and relative stenosis is defined as APD <13 mm [3,8]. Stenosis of the CSA of the spinal canal is defined as CSA-DS of <100 or <130 mm2, and lateral stenosis is defined according to thecutoff values of lateral recess height, as follows: 5 mm, normal (no stenosis); <3 mm, highly indicative; and <2 mm, diagnostic [8]. Many new suggestions have been made to define “stenosis” of the spinal canal and to identify the proper criteria.
To evaluate neurophysiologic deficits, electrodiagnostic methods such as dermatomal somatosensory-evoked potential (DSEP) studies can be used. DSEP offers the possibility of detecting neurophysiologic deficits in LSS, resulting in chronic compression of relatively long segments of dorsal rootlets [10,11]. Therefore, they can be used to provide additional information to the neurologic examination when LSS is suspected [12]. However, conventional electrodiagnostic studies have shown varying sensitivity and specificity using clinical reference standards and anatomic reference standards. Therefore, they showed no superior accuracy compared with MRI. This suggests that the evidence of DSEP for the diagnosis of LSS is still insufficient [4,13].
Nevertheless, DSEP was suggested to have a diagnostic value in LSS by representing chronic compression of a root in the spinal canal [10]. Eltantawi et al. [14] reported that DSEP had the highest electrophysiologic abnormality in patients with LSS and a good correlation with symptoms of neurogenic claudication; thus, DSEP had added to the clinical and radiologic assessment of patients with LSS by providing evidence for root dysfunction. Shen et al. [15] reported that the sensitivity and diagnostic concurrence with surgery of nerve root injury following LSS evaluated using DSEP was 95.7%, and the P40 latencies at L4, L5, and S1 in the case group were significantly longer than those in the control group. MRI is suggested as the most appropriate non-invasive test to confirm the presence of anatomic narrowing of the spinal canal or the presence of nerve root impingement [1]. Therefore, we assumed that there will be a significant correlation between neurophysiologic deficits in DSEP studies and anatomic narrowing in MRI in patients with LSS. We hypothesized that prolongation of the DSEP wave may be associated with narrowing of the spinal canal or neural foramen on MRI. The objectives of this study were to identify the difference of quantitative radiologic stenosis between a normal latency group and an abnormal latency group, and to investigate the association of L5 DSEP with quantitative radiologic stenosis on MRI in patients with LSS at the L4–5 disc level.
MATERIALS AND METHODS
Subjects
We retrospectively reviewed the medical records of patients with LSS at the L4–5 disc level. The inclusion criteria were as follows: unilateral buttock and leg symptoms with claudication confirmed as LSS on MRI, and electrodiagnostic tests including DSEP from January 2010 to December 2016 at the authors’ hospital. Ninety patients were identified. Fifty-one patients were excluded to eliminate the possibility of other causes having an influence on the conduction of the waves. The exclusion criteria were as follows: (1) history of lumbar surgery before MRI and DSEP studies (n=11); (2) presence of polyneuropathy such as diabetic polyneuropathy (n=26); (3) diagnosis of cervical or thoracic myelopathy (n=3); (4) history of spinal cord injury or stroke (n=2); (5) findings of more severe spinal stenosis in L3–4 and L5–S1 (n=6); and (6) APD >13 mm on MRI (n=3). We reviewed the demographic and clinical characteristics including age, sex, height, body mass index, and duration of LSS symptoms in 40 patients after exclusion. The study was approved by the Institutional Review Board of the Dongguk University Ilsan Hospital (No. 2016-52). The informed consent was waived.
DSEP study
We collected the P40 latencies of L5 DSEPs in both lower extremities. DSEP study was usually performed in L5 dermatomes of both legs to investigate the conduction abnormality (including demyelination and axonotmesis) in the spinal root caused by spinal stenosis at the L4–5 disc level. The DSEP study was performed with a certified machine (Medelec Synergy; Oxford Instruments, Surrey, UK) using a stimulation intensity double or triple to that of the sensory threshold with a pulse duration of 200 μs at a rate of <5 Hz. Stimulation was performed with the cathode located at either the level of the first metatarsophalangeal joint along its medial aspect or in the web space between the first and second digits of the foot. An anode was located about 2 or 3 cm distal to the placement of the cathode. The ground electrode was located on the ipsilateral patella. Recordings were performed at Cz’ and in reference to Fz on the scalp. Two sets of 128 responses were averaged to confirm the consistency of the waves in both lower extremities. P40 latency was detected from the waves for each patient. Instrument parameters were set with an analysis time of 100 ms and an amplifier sensitivity of 1 or 2 μV/div. We used two kinds of criterion of normal P40 latency. For the significant reference, P40 latency exceeding 51.0 ms was considered as delayed P40 latency [16] and a >3 ms difference between the latency on the affected side and the non-symptomatic side was considered to be an abnormal latency difference [17]. Because it has been reported that lumbar DSEP latency has a significant relationship to height, we used the following formula to standardize the data to the average height in our group for statistical comparison [18]:
Measurement of radiologic severity on MRI
The MRI examination was performed at 1.5 T using Siemens Avanto equipment (Siemens, Forchheim, Germany), and we selected the axial images (T2-weighted images of MRI) at the level of the L4–5 intervertebral disc for measuring central and lateral recess stenosis. The sagittal images (T2-weighted images) for the neural foramen on the affected side between the L5 and S1 level, where the L5 nerve root exits, were selected for assessing the CSA of the neural foramen (CSA-NF). Thereafter, we captured the images using the Picture Archiving and Communication System. The APD of the spinal canal, LID, and subarticular zone width (SZW) were measured by drawing a straight line between structures and were expressed in millimeters. The other parameters were measured by drawing their outlines with the regions of interest of the PiView (Infinitt, Seoul, Korea) program and were calculated as values in square millimeters.
On MRI, we measured the APD of the osseous spinal canal, CSA-DS, and LID to assess central stenosis; SZW to assess lateral stenosis; and CSA-NF to assess foramen stenosis [3,6,8]. LID was defined as the distance between the inner surfaces of the ligamentum flavum on a line connecting the joint space of facet joints at the level of the L4–5 intervertebral disc [7,8,19]. Its cutoff value for stenosis was 12 mm in one report and 15 or 16 mm in other reports [8]. With respect to lateral stenosis, SZW is the distance between the most anterior point of the superior articular facet and the posterior border of the vertebral body [20]. CSA-NF was measured on sagittal images below the pedicle. Because the nerve root is located more cranially than the lower end plate, no space below the line parallel to the lower end plate was included in area measurements [7,8] (Fig. 1).
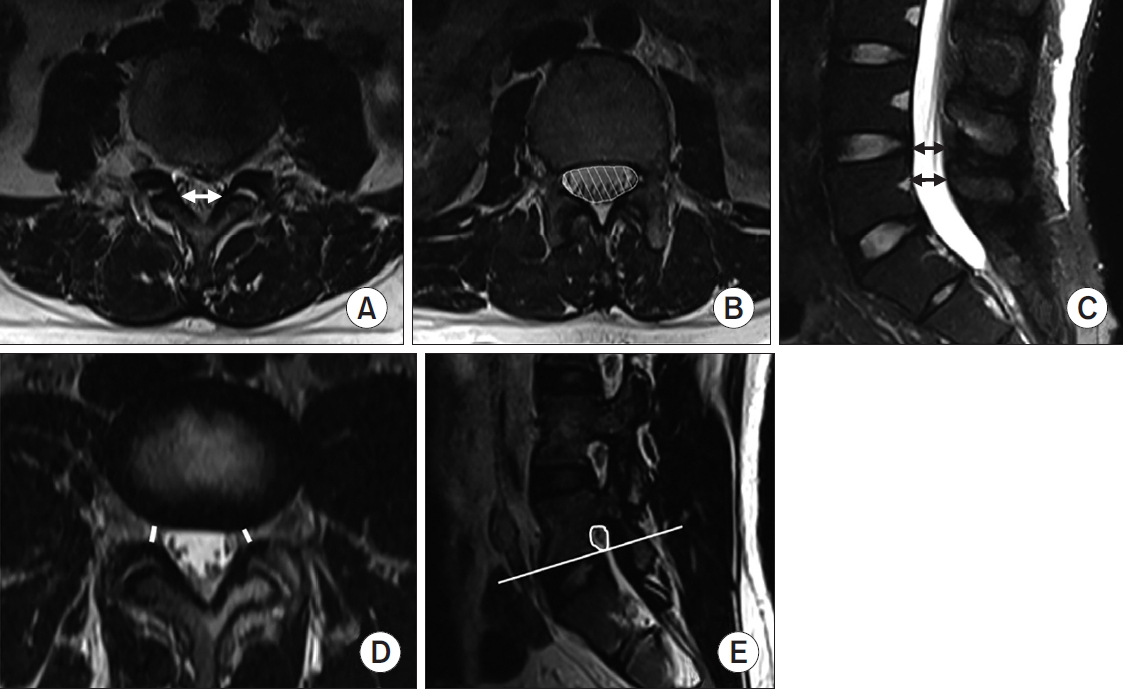
(A) The white arrow indicates the ligamentous interfacet distance measured between the inner surfaces of ligamentum flavum by connecting the joint space of facet joints. (B) Cross-sectional area of the dural sac is indicated by the white hatched area. (C) The black arrow indicates the anteroposterior diameter of the osseous spinal canal. (D) The minimal width of the subarticular zone was measured at the level of nerve roots using the disc, ligamentum flavum or facet joint as structures that constitute the borders of the neural canal. (E) The cross-sectional area of the neural foramen was measured on sagittal images below the pedicle. Because the nerve root is located more cranially than the lower end plate, no space below the line parallel to the lower end plate was included in area measurements.
Statistical analyses
We compared the differences in clinical characteristics and in radiologic narrowing between the normal and abnormal DSEP latency groups. For these comparisons, we performed the analysis using two reference values. We analyzed the correlation between standardized DSEP latencies and quantitative radiologic findings using Pearson correlation coefficient analysis in patients with absolute stenosis and those with relative stenosis, respectively. After the correlation analysis, backward stepwise regression for each factor, including clinical characteristics and radiologic parameters, was performed to establish the most influential factor for P40 latency. All statistical analyses were performed using SPSS-K version 20.0 (IBM SPSS, Armonk, NY, USA). The level of statistical significance was set at a p-value of <0.05.
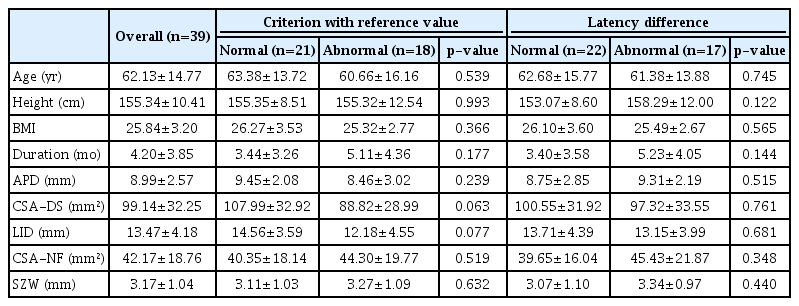
RESULTS
DSEP waves with P40 latency were evoked in 29 patients, and no wave was evoked in 10 patients. The average standardized P40 latency was 42.39±1.44 ms. Abnormal latency was found in 46% (n=18) of the patients. Comparison between the normal and abnormal latency groups with the reference value (P40 latency <51 ms) showed no significant differences in clinical characteristics and radiologic measurements. Abnormal latency difference was found in 44% (n=17) of the patients. Another comparison between the normal and abnormal latency difference groups also showed no significant differences (Table 1). In patients with relative stenosis of APD <13 mm, standardized P40 latency had a significant negative correlation with the APD (correlation coefficient [r]=-0.426, p=0.021) and LID (r=-0.494, p=0.006) of the spinal canal, which represent the morphologic severity of central stenosis. CSA-NF had a significant but weak positive correlation with standardized P40 latency (r=0.318, p=0.048). In patients with absolute stenosis of APD <10 mm, standardized P40 latency had a more significant negative correlation with APD (r=-0.539, p=0.007) and a less significant negative correlation with LID (r=-0.459, p=0.024). However, P40 latency had no significant correlation with CSA-NF (r=0.363, p=0.081) (Table 2). In backward stepwise regression analysis between P40 latency and each factor in all patients with evoked waves, LID was shown to be the only significant factor (regression coefficient [β]=-0.930, p=0.011).
Comparison of clinical characteristics and radiologic narrowing between the normal latency group and abnormal latency group
DISCUSSION
To the best of our knowledge, there is no article on quantitative analysis and direct correlation between DSEP and stenosis parameters of MRI in patients with LSS. The diagnostic criteria for LSS are uncertain and consensus among experts is not confirmed. Moreover, imaging studies such as MRI can be expected to indicate the morphologic severity of stenosis. However, the diagnostic validity is unclear. Therefore, the diagnostic value of DSEPs is worth investigating because a delay in DSEP latency can be assumed to physiologically reflect stenosis around the spinal nerve root in the central spinal canal and neural foramen. Therefore, we expected a significant difference in MRI parameters for spinal stenosis between the groups categorized by the severity of DSEP findings, such as the prolongation of latency itself and the difference in latency on the symptomatic and asymptomatic sides. Additionally, we expected that the prolongation or abnormality in DSEP wave latency will have a significant negative correlation with the morphologic severity of stenosis on MRI, which means that the more severe is the stenosis, the more delayed would be the P40 latency. However, the comparison of radiologic parameters between the normal latency group and the abnormal latency group did not show any significant results. Therefore, when diagnosing LSS based on imaging modalities, the additional role of DSEPs seems less valuable. In fact, the diagnostic value of DSEPs is still controversial. The results of this study showed abnormal latency in 46% (n=18) of all included patients and an abnormal difference in latency in 44% (n=17) of all included patients.
DSEP study was suggested to be valuable as an addon diagnostic procedure to imaging studies [14], as well as to be a useful supplementary test for the diagnosis of LSS [21]. Eltantawi et al. [14] reported the high sensitivity of abnormal waves of DSEPs through a comparison with a control healthy group and set clinical symptoms of claudication as a comparative standard. However, they compared only two variables (normal and abnormal latencies without real latency) and did not analyze quantitative data. In patients with LSS, another study reported that tibial somatosensory-evoked potentials (SEPs) were delayed in 78%, pathologic compound muscle action potential reductions were found in 39%, and a pathologically prolonged H-reflex was found in 52%. That study included patients with symptoms in both limbs [12]. A recent review reported that the diagnostic accuracy of DSEPs remains unclear despite the high sensitivity because of the small number of studies and the use of different reference standards. The diagnostic accuracy of DSEPs was reported to have a high or modest sensitivity (78%–94%), with no reports about the specificity. Overall, the diagnostic accuracy of other electrodiagnostic tests (electromyography [EMG], nerve conduction study, magnetic stimulation-induced motor conduction time, and selective lumbar root sheath infiltration) was only modest. Paraspinal mapping had a high specificity in two studies and may increase the likelihood of diagnosing LSS. The authors concluded that these electrodiagnostic studies showed no superior accuracy for conventional electrodiagnostic testing compared with MRI [6]. Another recent study reported that needle EMG showed a significant correlation with clinical symptoms, whereas MRI findings did not show a significant correlation in patients with lateral stenosis on MRI. The authors suggested that EMG findings can physiologically reflect stenosis around the nerves, but MRI parameters are not sufficient to explain the multifactorial causes of LSS [22].
However, when we analyzed the correlation between standardized DSEP latencies and quantitative radiologic findings in patients with evoked waves, the APD of the spinal canal and the LID showed significant negative correlations with the P40 latency in both the relative central stenosis and absolute central stenosis groups. In backward stepwise regression for each factor, we observed that LID was the only significant factor affecting P40 latency. We suggest that P40 latency seems to be affected by central stenosis, especially because of ligamentum flavum thickening, which is related to central stenosis. Further, it can be suggested that ligamentum flavum thickening has a greater influence on the dorsal root transporting sensory signals than on the ventral root; thus, it can affect the latency of P40 SEP waves. Another study with 54 patients undergoing decompressive spine surgery after the diagnosis of LSS reported that there was no significant correlation between tibial SEPs and the CSA-DS and the osseous spinal canal on MRI [12]. Liu et al. [18] reported that lumbar SEP study, which was performed with recording at the T12 spinous process and was suggested to be well restricted to the lumbar region, is able to effectively detect neurologic deficits in the lumbar area in patients with LSS. They supposed that cortical SEPs, which are the most commonly used, cannot reflect the true neurologic condition of the lumbar spine and are not very useful in clinical assessment. The findings of these reports are opposite to our results; however, these studies did not include LID as a parameter of central stenosis. Conversely, the results on the relationship between P40 latency and lateral stenosis parameters were beyond our expectation. CSA-NF showed a weak significant positive correlation with P40 latency (p=0.048) only in relative spinal stenosis, but showed no significant correlation in absolute, more severe, spinal stenosis. We supposed that these findings possibly resulted from technical errors, because measuring neural foramen stenosis was more difficult than measuring central stenosis and lateral stenosis. CSA-NF was measured on sagittal images below the pedicle [20]. Hence, it was rather difficult to select the exact sagittal image cut representing the foraminal zone. Moreover, as the nerve root is located more cranially than the lower end plate, no space below the line parallel to the lower end plate was included [19]. In fact, CSA-NF is not a generally accepted radiologic parameter for lateral stenosis [23]. Semi-quantitative or qualitative radiologic criteria such as the grading system according to the amount of perineural intraforaminal fat, hypertrophic facet joint degeneration, compression of the foraminal zone, foraminal nerve root impingement, and size and shape of the foramen havebeen reported. The grading system of perineural intraforaminal fat showed good reliability, but the other criteria showed variability in intrarater and inter-rater reliability [24]. With respect to SZW, it was measured by drawing a line between the most anterior point of the superior articular facet and the posterior border of the vertebral body [19]. This measurement requires a rather delicate process because even a slight error in measurement may severely affect the latency, as its mean±standard deviation was 3.17±1.00 mm (Table 1). If a larger number of patients are involved, the impact of a single technical error would be reduced.
This study had some limitations. First, there is no gold standard method for the diagnosis of spinal stenosis. Surgical findings and the clinical results after surgery could be the best diagnostic criteria; however, there were 10 patients who had undergone spinal surgery after the diagnosis and we could not perform the analysis according to surgical findings. Second, this was a retrospective study with insufficient clinical information. As the diagnosis of spinal stenosis was usually based on the clinical impression, the analysis of the relationship of DSEP to clinical characteristics such as pain intensity, claudication severity, and functional scales, such as Oswestry Disability Index, can be valuable. However, because it was a retrospective review, we could not obtain such clinical information and additional analyses including clinical factors, latency of DSEPs, and morphologic severity are needed to identify the diagnostic usefulness of DSEPs in patients with LSS. Third, the number of patients for the analysis was small. We did not routinely perform DSEP studies based on the impression of spinal stenosis or herniated disc disease because of the low sensitivity of needle EMG and nerve conduction study. We only performed a DSEP study in cases in which there was a strong impression of spinal stenosis or a referral from spine surgeons to identify the involvement of the root in LSS. Therefore, the number of patients with DSEP data was small. Fourth, there was no segmental study. Liu et al. [18] reported that lumbar SEPs are able to effectively detect neurologic deficits in the lumbar area in patients with LSS, and cortical SEPs cannot reflect the true neurologic condition of the lumbar spine. However, another study comparing segmental mixed nerve SEPs with DSEPs reported that S1 and L5 DSEP latency showed higher sensitivity and specificity and relatively lower sensitivity and specificity in N25 and N45 latency, and poor sensitivity and specificity in N20 and N10 latency of tibial nerve mixed SEPs [21]. We only performed cortical SEP study with P40 latency, which represents the function of the long sensory tract from the foot to the cortex, because we experienced difficulty in obtaining waves in the segmental study with simultaneous recording at the popliteal fossa, lumbar spine, thoracic spine, and scalp. We attempted to exclude diseases that may have an influence on the long sensory tract, such as myelopathy and distal sensorimotor polyneuropathy.
In conclusion, despite the lack of differences in the radiologic severity of LSS between the normal and abnormal DSEP groups, the latency of DSEPs had a negative correlation with the severity of central stenosis and LID was an influencing factor in the delay of DSEP latency. Further studies about the association between clinical factors and DSEP and the diagnostic value of DSEP will be required.
Notes
No potential conflict of interest relevant to this article was reported.
Acknowledgements
This work was supported by the Dongguk University Research Fund of 2016. Special thanks are due to the biostatistician (Chi-Yeon Lim, PhD) of the Department of Data Management and Statistics Institute, Dongguk University.
Notes
Conceptualization: Lee HJ. Methodology: Lee HJ, Yang DC, Nam K, Cho KT. Formal analysis: Lee HJ, Yang DC, Park JW. Investigation: Nam K, Yang DC, Kim S. Resources: Lee HJ, Nam K, Park JW. Writing - original draft: Yang DC. Writing - review and editing: Lee HJ. Supervision: Kwon BS. Project administration: Lee HJ. Funding acquisition: Lee HJ. Approval of final manuscript: all authors.