- Search
| Ann Rehabil Med > Volume 40(1); 2016 > Article |
Abstract
Axial mesodermal dysplasia complex (AMDC) arises in variable combinations of craniocaudal anomalies such as musculoskeletal deformities, neuroschisis, or rhombencephalic developmental disorders. To the best of our knowledge, the co-existence of AMDC with associated musculoskeletal anomalies, medullary neuroschisis with mirror movements, and cranial nerve anomalies has not yet been reported. Here, we report the case of a 4-year-old boy whose clinical features were suggestive of Goldenhar syndrome and Poland syndrome with Sprengel deformity. Moreover, he showed mirror movements in his hands suspected of rhombencephalic malformation, and infranuclear-type facial nerve palsy of the left side of his face, the opposite side to the facial anomalies of Goldenhar syndrome. After conducting radiological studies, he was diagnosed with medullary neuroschisis without pontine malformations and Klippel-Feil syndrome with rib anomalies. Based on these findings, we propose that clinical AMDC can be accompanied by a wide variety of musculoskeletal defects and variable degrees of central nervous system malformations. Therefore, in addition to detailed physical and neurological examinations, imaging studies should be considered in AMDC.
Axial mesodermal dysplasia complex (AMDC) is a combination of various malformations of musculoskeletal structures and internal organs, including the branchial, cardiovascular, pulmonary, gastrointestinal, and genitourinary system, which is caused by abnormal fetal mesodermal development [1]. As suggested by the definition, AMDC can occur as a wide spectrum of clinical manifestations ranging from rostral craniofacial anomaly to caudal sacral deformity including multiple forms of axial skeletal anomalies. Owing to this clinical manifestation of anomaly, AMDC includes many syndromes such as Goldenhar, Klippel-Feil, VACTERL, and MURCS [2]. Among these clinical syndromes, a few have been reported to be associated with central nervous system (CNS) anomalies. Klippel-Feil syndrome, which was recently classified as AMDC [1], has been widely recognized as a cervical vertebral anomaly and is often associated with cervical spinal cord anomaly including cervicomedullary junction, and sometimes mirror movements [3,4]. Similarly, there have been several reports of Goldenhar syndrome accompanied by CNS dysplasia, including not only facial skeletal deformities but also cranial nerve anomalies [5,6]. Taken together, in the AMDC syndromes, CNS malformations appear to be frequently enrolled. Thus, AMDC could be considered as a complex of deformities that occurs as a combination with not only musculoskeletal deformities, but also CNS developmental disorders. In line with this suggestion, here, we report a case of a complex form of AMDC involving musculoskeletal deformities such as right facial deformities, submucosal cleft palate, cervical spine and rib malformations, right scapular deformities, and the rhombencephalic anomaly of medullary neuroschisis with left infranuclear-type facial nerve palsy.
The patient, a 4-year-old boy, was referred to the rehabilitation medicine department for evaluation of facial deformities and posture abnormalities. He was born prematurely at 35 weeks via cesarean section due to the premature rupture of fetal membranes. The mother was healthy and did not report the use of alcohol, tobacco, or drugs during pregnancy. The family history was non-contributory. At birth, it was noticed that he had a congenital cardiac defect; ventral septal defect, atrial septal defect, and patent ductus arteriosus, for which he underwent surgical treatment 6 months after birth. According to his mother's description, he was otherwise healthy with normal development. After performing a careful physical examination, several dysmorphic features were noted including asymmetrical facial skeleton, left facial weakness, and right microtia (Fig. 1A). Additionally, uvular bifida was noticed when the inside of his mouth was examined (Fig. 1B). His neck was relatively short, the right shoulder blade was located relatively more superior than the left, and the range of motion of the shoulder was limited during abduction (Fig. 1C). Moreover, the right pectoralis major appeared shrunken, and thoracic asymmetry was confirmed, where the right nipple was relatively lower than the left (Fig. 1D). In addition to these malformations, the mirror movements of the opposite hand during voluntary movements of each hand were observed. Additional neurological examination of the patient showed left facial palsy, but no ocular movement disorder was observed. Furthermore, muscular strength of the limbs was normal, and pathological reflexes and upper motor neuron signs were not observed.
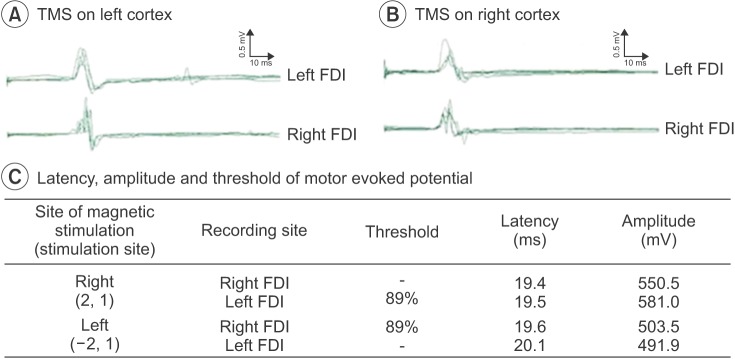
According to the Korean-Wechsler Intelligence Scale administered to evaluate the cognitive function of the patient, verbal intelligence quotient (IQ) index was 71, performance IQ index was 86, and the overall IQ index was 76. Cervical spine and brain magnetic resonance imaging (MRI), and cervical and thoracic computerized tomography (CT) were performed to rule out the additional CNS malformations and musculoskeletal anomalies. Cervical spine and brain MRI showed medullary neuroschisis without pontine or cerebellar hypoplasia, and there was no evidence of abnormalities of descending tracts and transpontine fibers in the basis pontis in the diffusion tensor MRI (Fig. 2). In the cervical spine, vertebral body fusion at the level of C3 and C4 was observed (Fig. 2C), and cervical and thoracic CT images showed dysplasia at the posterior cervical spine (Fig. 3A–D). In addition, fusion deformities of the right 2nd and 3rd rib, incomplete development of the right 1st rib were observed (Fig. 3E), and the right scapula was elevated compared with the left (Fig. 3F). To reveal the motor organization pattern of both hands associated with mirror movements, motor evoked potentials (MEP) study was performed with transcranial magnetic stimulation. The optimal stimulation coordinate was selected if a MEP of 50 µV or more was evoked at least 5 times from 10 stimulations of transcranial magnetic stimulation with the lowest excitation threshold. The optimal stimulation position for the left cortex was (–2,1) according to the 10–20 system, MEPs were simultaneously evoked at the right and left first dorsal interossei muscles; the threshold was 89% of the maximum stimulus intensity. Similarly, the optimal stimulation position for the right cortex was (2,1) MEPs were simultaneously evoked at bilateral first dorsal interossei muscles (Fig. 4). Facial motor nerve conduction studies showed a delayed latency and low amplitude of the left facial nerve compared with the right, where facial skeletal deformities were observed.
This report describes a patient who simultaneously showed musculoskeletal deformities of AMDC such as unilateral facial skeletal deformities, submucosal cleft palate, cervical spine deformities, and scapular anomaly, congenital heart disease, and the rhombencephalic anomaly; medullary neuroschisis with associated mirror movements and unilateral facial palsy. The patient described herein presented with various defects extending to the craniocaudal level with various gradations of severity, and a wide range of CNS malformations, which were detected by physical and neurological examinations, radiological findings, and electrophysiological evaluations. These occurrences of various combinations and the various severity gradations for congenital anomalies have not yet been reported from a Klippel-Feil syndrome 'plus' point of view [7]. We think that from a Goldenhar syndrome point of view, this co-occurrence of CNS malformations; rhombencephalic anomaly in Goldenhar syndrome may also be a very unusual case.
From the present case, we suggest that these complex malformations with various gradations of severities reflect sequential disruption of the tissues derived from one or more of three germ layers during embryogenesis. Generally, deformities accompanying AMDC are believed to arise from the failure of mesodermal cell migration around the 4th week of embryogenesis [2]. However, this prevailing hypothesis has a limitation in explaining the pathogenic mechanism of this case, because rhombencephalic malformations, which arise from abnormalities in neuroectodermal development, cannot occur in this period. In particular, the co-existing dysplasia of the musculoskeletal system including cranial and postcranial regions and cranial nerve system may have been derived from sequential disruption of the tissues during extended periods from the early stages of neural tube formation to the period of neuroectodermal differentiation. From previous studies of the period of the occurrence of these anomalies, we found that cervicomedullary neuroschisis occurs during neural tube formation [8], and brainstem anomaly below pons appears to occur during rhombencephalic development [6,9]. A previous study reported that neuroschisis at the level of the cervical spine, induced by neural tube malformation, may affect the sequential development and migration of neural crest cells after neural tube formation, and may induce anomalies in musculoskeletal and CNS maldevelopment [2]. Moreover, another previous study regarding Mobius syndrome with musculoskeletal deformities, which is believed to be a complex developmental disorder in the period of the 3rd and 8th week of embryogenesis [10], is in accordance with our observation. Considering these previous results, the present case of dysplasia may result from a failure in neural tube fusion and mesodermal cell migration, which takes place during the 3rd to 4th week of gestation, and the failure of sequential rhombencephalic segmentation after neural tube formation in the 5th week, as well as the abnormal differentiation of the metencephalon and myelencephalon, especially myelencephalon dysplasia. From the point of view of the various combinations and the gradations of anomalies, we can deduce that when abnormalities occur during the vulnerable stages of embryonic development (e.g., neural tube formation, mesodermal migration, and rhombencephalon segmentation), discrete or continuous associated developmental disabilities can occur. Depending on the severity of damage and the ability of the embryo to restore itself, phenotypes after birth can be accompanied by abnormalities of different degrees in internal and external structures of mesodermal origin. We cannot conclusively exclude the AMDC spectrum with dysplasia of the CNS. Therefore, when examining patients with sporadic phenotypes of AMDC, hidden axial mesodermal dysplasia must be closely observed, and neurological screening including CNS examination should be performed simultaneously. In addition, we also suggest that the neurophysiological mechanism of the hind brain abnormality, the medullary neuroschisis, which could lead to the abnormal pyramidal decussation, may induce the neural substrate for the motor organization of the bilateral mirror movements. In accordance with this suggestion, a previous study revealed an association between cervicomedullary neuroschisis and mirror movements in Klippel-Feil syndrome patients [4]. Therefore, we can propose that the unknown origins of congenital mirror movements may be the result of developmental abnormalities at the cervicomedullary junction occurring as early as the embryonic period.
In conclusion, cases of AMDC with minor sporadic dysplasia should be examined for various anomalies for musculoskeletal structure and internal organs or dysplasia of neural structures that cannot be noted through simple physical and neurological examination. Among the various abnormalities accompanying AMDC, CNS malformations can be encountered. Since CNS malformations with mild symptoms can be easily overlooked, neurological examination, including brain imaging, should be recommended for patients suspected of having AMDC. Further studies on larger cohorts are needed to reveal the underlying cause of this syndrome and to better understand its pathological mechanism.
CONFLICTS OF INTEREST
CONFLICT OF INTEREST: No potential conflict of interest relevant to this article was reported.
References
1. Stewart FJ, Nevin NC, Brown S. Axial mesodermal dysplasia spectrum. Am J Med Genet 1993;45:426-429. PMID: 8465843.


2. Bergmann C, Zerres K, Peschgens T, Senderek J, Hornchen H, Rudnik-Schöneborn S. Overlap between VACTERL and hemifacial microsomia illustrating a spectrum of malformations seen in axial mesodermal dysplasia complex (AMDC). Am J Med Genet A 2003;121:151-155. PMID: 12910495.

3. Dias MS, Walker ML. The embryogenesis of complex dysraphic malformations: a disorder of gastrulation? Pediatr Neurosurg 1992;18:229-253. PMID: 1476931.


4. Royal SA, Tubbs RS, D'Antonio MG, Rauzzino MJ, Oakes WJ. Investigations into the association between cervicomedullary neuroschisis and mirror movements in patients with Klippel-Feil syndrome. AJNR Am J Neuroradiol 2002;23:724-729. PMID: 11950676.


5. Preis S, Majewski F, Hantschmann R, Schumacher H, Lenard HG. Goldenhar, Mobius and hypoglossiahypodactyly anomalies in a patient: syndrome or association? Eur J Pediatr 1996;155:385-389. PMID: 8741036.


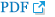
6. Huang HT, Hwang CW, Lai PH, Chen CC. Mobius syndrome as a syndrome of rhombencephalic maldevelopment: a case report. Pediatr Neonatol 2009;50:36-38. PMID: 19326837.


8. Muroi A, Fleming KL, McComb JG. Split medulla in association with multiple closed neural tube defects. Childs Nerv Syst 2010;26:967-971. PMID: 20179945.


9. Webb BD, Frempong T, Naidich TP, Gaspar H, Jabs EW, Rucker JC. Mirror movements identified in patients with Moebius syndrome. Tremor Other Hyperkinet Mov (N Y) 2014;4:256PMID: 25120946.



10. Guebert GM, Rowe LJ, Yochum TR, Thompson JR, Maola CJ. Congenital anomalies and normal skeletal variants. In: Yochum TR, Rowe LJ, editors. Essentials of skeletal radiology. 1st ed. Baltimore: Lippincott Williams & Wilkins; 1987. p.95-168.
Fig. 1
Patient showing clinical features consisting of microtia of the right ear, which is quite lowset (white arrow) (A) and uvula bifida (white arrow) (B). Patient showing congenital migration of the scapula superiorly compared with the thoracic cage (white arrow) (C). Patient showing asymmetric thoracic wall: agenesis of the right pectoralis major muscle and hypoplastic right nipple (white arrow) (D).
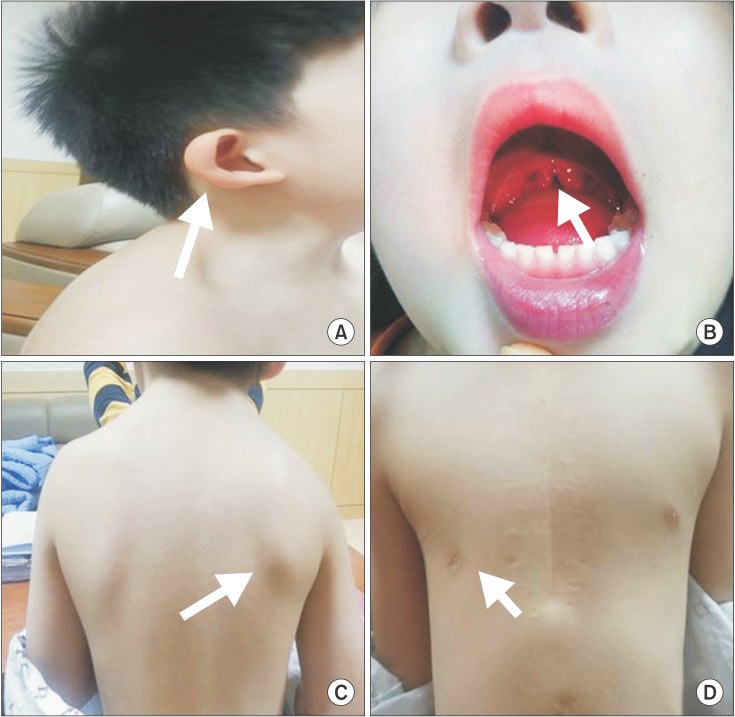
Fig. 2
T1-weighted transverse sagittal magnetic resonance image showing neuroschisis at the cervicomedullary junction (white arrow) (A) and split-cord malformation at the upper cervical cord (white arrowheads) (B). T1-weighted sagittal magnetic resonance image showing a cervical vertebral fusion at the level of C3–4 (white arrowhead) (C). The corticospinal tract (CST), central tegmental tract (CTT), and the transverse pontine fibers between CST and CTT on diffuse tensor imaging, with color fractional anisotropy maps in the red circle at the upper pontine level being normal (D).
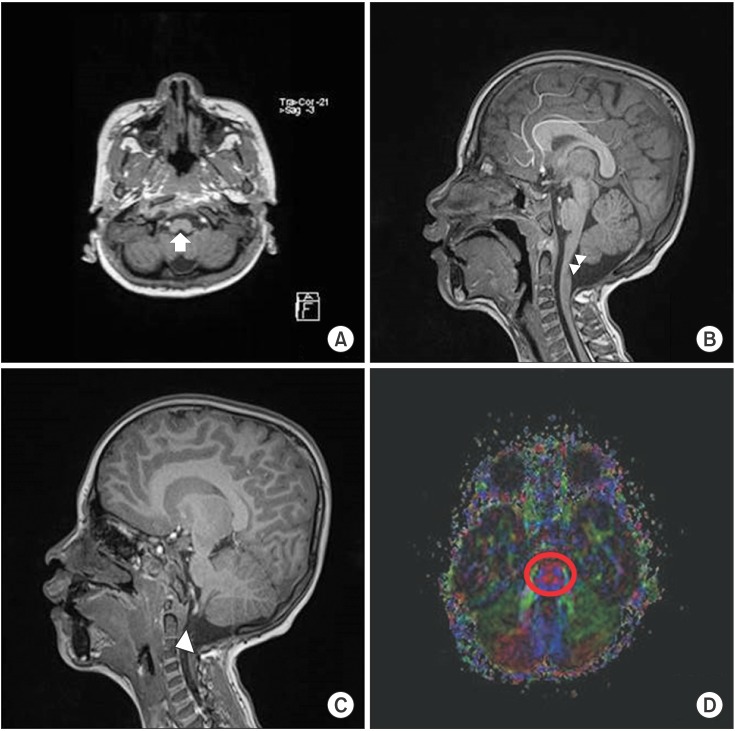
Fig. 3
Transverse c-spine computed tomography (CT) images showing aplasia of the C1 anterior and posterior arches (red arrows) (A), aplasia of the C2 posterior arch (blue arrow) (B), and the C3 left laminar aplasia (yellow arrow) (C). A simple lateral c-spine X-ray image showing the complete absence of the post arch of the C1 and C2 spine (white arrows) (D). 3D CT-reconstruction image showing right 1st rib agenesis (white arrow) and right 2nd and 3rd rib fusion (white arrowhead) (E). 3D CT-reconstruction image showing that the right scapula migrated superiorly compared with the left, and the inferior angle of the scapular blade is at the level of the 6th rib (white arrows) (F).
Fig. 4
Motor responses from transcranial magnetic stimulation (TMS) showing bilateral motor-evoked potentials from the first dorsal interosseous (FDI) muscles during left motor cortex stimulation (A). Motor responses of TMS showing bilateral motor-evoked potentials from the FDI muscles during right motor cortex stimulation (B). The best stimulation position was (–2,1) in the left cortex and (2,1) in the right cortex, and the threshold was 89% at both cortices (C).
- TOOLS
-
METRICS

- Related articles in ARM
-
Arnold-Chiari Malformation Type III With Meningoencephalocele: A Case Report2014 June;38(3)





